

(Q-1) An electron splits into everywhere in 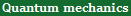
Quantum mechanical molecular bonds lack reality.
See this week physics is still useless.
(P-1) ↓ Photoelectric effect = electron absorbs "virtual"  ?
?
Einstein's famous photoelectric effect is said to prove light can behave like a particle called a "photon".
But the photoelectric effect has nothing to do with fantasy "photon".
It just demonstrated an electron absorbing the ordinary classical light wave with "short wavelength" (= hence, wave nature of light ! ) is ejected from an atom.
The truth is that it is physically impossible for an electron to absorb or emit a real photon in photoelectric effect. So Einstein is false.
We all know the total energy E (= the sum of kinetic + potential energies of all particles ) is always conserved.
At the same time, the total momentum p (= product of a particle's mass m times its velocity v ) is always conserved, too.
Photoelectric effect says when an electron at rest (= kinetic energy is zero ) absorbs a photon with energy E, the electron's kinetic energy increases by exactly the same amount E as the photon's energy the electron absorbs, and the 'juiced up' electron starts to move.
According to the total energy conservation law, the magnitude of the incoming photon energy E must be just equal to the magnitude of the resultant kinetic energy E of the electron which absorbs the whole photon.
The total momentum must be conserved, too. So the incoming photon's momentum p must be equal to the resultant electron's momentum pe ( p = pe ).
But this momentum can never be conserved, though it must be conserved according to the physical principle.
Because the ratio (= E/p ) of energy (= E ) to momentum (= p ) is different between the photon and the electron, which fact makes it impossible to conserve both energy and momentum at the same time.
The incoming photon's momentum (= p ) is equal to the photon's energy E divided by the photon (= light ) speed c ( p = E/c ).
On the other hand, an electron is known to have the kinetic energy E (= E = 1/2mv2 where m is electron's mass, v is electron's velocity ) and momentum p (= p = mv ).
The momentum (= pe = mv ) of the resultant electron absorbing photon's energy E ( photon energy E is transferred to electron's kinetic energy E = 1/2mv2 = 1/2pev where momentum pe = mv ) is equal to 2E divided by the electron's speed v ( pe = 2E/v ), as shown in the upper figure.
The light speed c is much higher than the electron's speed v ( c > v ), so the photon's momentum p is much smaller than the electron's momentum pe ( p < pe ), when their energy E is the same ( photon's momentum p = E/c is much smaller than electron's momentum p = 2E/v because the light speed c is much bigger than electron's velocity v while transferred energy E is the same ).
↑ The total momentum between photon and electron is Not conserved (= photon's momentum is much smaller than the electron's momentum ), when total energy E is conserved.
As a result, it is impossible to conserve both total energy and momentum in Einstein photoelectric effect where an electron absorbing a photon obtains kinetic energy and escapes from an atom. ← Einstein's photon violates basic physical rule (= energy-momentum conservation ), so unreal.
The total energy and momentum must be always conserved (= according to all experimental results ).
If the total energy and momentum between incident photon and the ejected electron in photoelectric effect are conserved, the photon must be unreal virtual photon with imaginary mass which contradicts Einstein relativistic mc2 relation ! ← So the present mainstream physics is contradictory and false.
Einstein ad-hoc relativistic theory dismissed the existence of "medium" filling space. So the space constains "nothing" according to Einstein.
In this Einstein empty space, there are neither 'guideposts' nor beacons which tell us where and how fast each particle is actually moving on the "blank" space map.
So in Einstein's uncanny space, there is no such thing as "absolute" space. All things are "relative", any physical properties such as velocity and kinetic energy are undetermined and varying, seen by different observers.
An electron at rest (= ① in upper figure ) absorbs a photon, acquires kinetic energy and starts to move with speed v (= ② ) rightward seen by an observer at rest with respect to the stationary electron ①.
But seen by another observer moving with speed v rightward (= lower part in this figure ), the initial electron ① appears to be moving leftward (= instead of 'at rest' ) at speed v, and then stop after absorbing a photon energy. ← This supernatural phenomenon can never happen in real world.
Because this moving observer witnesses paradoxical situation where an electron, which should increase its (kinetic) energy after absorbing photon energy, does the 'opposite thing' = decreases its kinetic energy and stops after obtaining the photon's energy E (= ② in lower part of this figure ) !
This paradoxical situation is caused by Einstein's unrealistically "empty" space or "blank" map.
In fact, the electron's kinetic energy and momentum are intrinsically "relative" concepts with respect to "other external things" such as "medium" or "beacon" interacting with electron.
Therefore, there is No such thing as "absolute kinetic energy" or momentum. The situation where an electron "actually has" some amount of kinetic energy ( or momentum ) in the completely empty space is impossible.
So in the empty space favored by Einstein relativity, it is impossible for an electron to absorb a photon, "obtain" new (kinetic) energy and momentum in photoelectric effect. ← This sounds strange, but it is true.
As a result, the current physics "gave up reality", and started to say an electron cannot emit a real photon, only "unreal virtual photon" can "supernaturally" interact with an electron in photoelectric effect and electromagnetic force.
Physicists use nonphysical, abstract Feynman diagram to describe interaction between electrons and photons.
In this Feynman diagram, both energy and momentum must be conserved.
Using this energy-momentum conservation law and Einstein relativistic equation, we find virtual photon (= absorbed or emitted by an electron ) must have unphysical "imaginary" mass (= virtual photon's mass squared can unrealistically become negative, m2 < 0, this p.3 ).
This virtual photon is obviously a self-contradictory concept, and shows Einstein relativistic "photon" is wrong, does Not really exist, because virtual photon cannot satisfy Einstein relativistic energy-momentum equation ( this p.5 ).
So Einstien's photon is just literally 'virtual' without reality.
Photoelectric effect described by the current quantum mechanics and Einstein must rely on this "unreal virtual particles", hence all the current mainstream theories lack reality due to their inherent fatal self-contradiction.
All phenomena such as light interference, refraction, "light wavelength change" in Compton scattering show light is actually "wave" traveling through some "medium".
So all the actually measured physical properties such as wavelength, energy, momentum of light, sound, electron's de Broglie wave are generated with respect to interacting with some "external medium" as "beacon", instead of Einstein's absolute empty space or blank map.
If we believe in Einstein's ad-hoc theory where there is no such thing as "real medium" in space, a single electron has to pass through two slits simultaneously to inferfere with itself using illusory parallel worlds.
Without real medium in space, it causes "de Broglie wave paradox".
A stationary observer actually sees de Broglie wave interference of an electron, while another observer moving at the same speed as an electron, the electron's speed v appears to be zero, = No electron's de Broglie wave(length) is generated, hence, electron's de Broglie wave interference pattern appears to disapper or reappear seen by a moving or a stationary observer. ← Einstein paradox !
Physicists just roughly treat "light wave" with energy enough to kick out an electron from an atom as a fictitious "photon" without specifying any detailed shape or size of an imaginary single photon.
In uncanny quantum mechanics, each electron always has to spread all over space as "electron probability cloud" mixing up electron's particle and wave pictures as one nonphysical wavefunction.
So the clear separation of a "particle" from "wave" is impossible in quantum mechanics, where an electron particle exists everywhere simultaneously using its nonphysical "probability" wave nature = fictional parallel worlds.
Inside this strange quantum mechanical foggy electron cloud, an electron cannot realistically avoid other negative electrons or approach other positive nuclei. ← Coulomb force is Not normally working, ← distance between electrons is disorderly, random ← Total energy can Not be conserved ← Hence, finding exact solutions which give conserved (= constant ) total energy value E in Schrödinger equation of any multi-electron atoms is impossible.
So unrealistic quantum mechanical electron cloud has to rely on some "dirty tricks" to generate (fake) molecular bond energy by violating total energy conservation law and lowering kinetic energy illegitimately, because real Coulomb force is unavailable .
To treat atomic, molecular structures and Coulomb forces realistically (= without relying on quantum mechanical parallel worlds ), we need to separate an electron "particle" from its de Broglie "wave" generated in the "medium" around the electron.
Unfortunately, this realistic atomic model which must calculate complicated, dynamic multi-electron motions (= three-body problem ) was impossible in 1920s when there were No modern computers and physicists had no choice but to accept unrealistic quantum mechanical model.
If an electron tries to absorb or emit a photon (= a particle of light ) in the Einstein empty space, this photon must be an unreal virtual photon with nonphysical imaginary mass, when the total energy and momentum are conserved between the electron and the fictitious photon.
Hence, the only realistic solution is a real electron needs to absorb or emit "light wave" through friction against the external "medium" around the electron to conserve total momentum and energy safely without relying on self-contradictory virtual photon.
When an orbital length of a moving electrons is not an integer times de Broglie wavelength, an electron crashes into its destructive de Broglie wave (= friction against the surrounding medium ) and loses energy, emitting electromagnetic wave in the direction perpendicular to the initial electron moving (= crashing ) direction.
Each electron is orbiting around a heavy nucleus, bound by nuclear positive charge (= so the total momentum of an electron and a nucleus bound together is always almost zero, regardless of their total energy values ).
The "crashing energy" between a moving electron and its destructive de Broglie wave (= these two crashing things have about the same magnitude of momentum, move in the opposite direction and crash into each other, so their resultant total momentum is zero ) is stored as medium's "potential ( spring-like ) energy" which soon becomes the "oscillating energy" in the transverse (= crashing ) direction (= this energy is detected by photo-electric effect ).
This crashing energy oscillating in the transverse direction is pushed out from the point where an moving electron crashes into the opposite de Broglie wave in the perpendicular direction (= longitudinal = light traveling direction in which momentum is detected by Compton scattering ) as "electromagnetic wave".
This resultant light wave has small momentum in longitudinal (= light traveling ) direction, and high oscillating energy in the transverse (= electron's crashing ) direction, which is compatible with light's high energy compared to low momentum, hence, safely conserves both energy and momentum in interaction with an electron with different ratio of energy to momentum.
So using real moving electrons, de Broglie wave generated around them and light wave actually traveling in the medium with various wavelength, we can explain all atomic bahavior without self-contradiction, quantum mechanical parallel-worlds or paradoxical virtual photons.
But as long as the obsolete useless quantum mechanics is used, physicists have to rely on unreal quasiparticle model, faster-than-light spin and bogus one-electron DFT approximation, whenever they treat many-electron materials or solid-physics to interpret the measured physical phenomena.
And physicists needed to make up "illusory scientific goals" such as parallel-world quantum computer, information, God-particle, nonphysical symmetry to deceive governments and continue to get research fund from taxpayers' money, while the current old-fashioned school system is 'harmful' rather than beneficial, indoctrinating students with fake science.
QED method using these unreal virtual particles is used only for negligibly tiny values such as g-factor and Lamb shift which are too small to be useful for our daily life, so meaningless scientific area.
Actually, No current physicists use this useless QED with virtual photons in practical science or daily life. ← It means it is safe to say all these ad-hoc QED tricks are unnecessary, unreal and can be replaced by (= incorporated into ) other more general and realistic explanation or theory.
We have No trouble even if there were No virtual particles (= which just include self-contradiction, so false ) and ad-hoc QED interpretation of negligibly tiny values is replaced by other more realistic factors such as some "artifact" or multi-charged particles' tiny fluctuating motion without unreal virtual particles.
QED calculation results always diverge to "infinity" due to infinite unreal virtual particles' effect ( this p.4 ). So it just artificially subtracts "arbitrary infinity" from the "infinite calculation result" by illegitimate tricks called "renormalization" to get meaninglessly tiny (= freely-adjustable by QED trick ) physical values which are useless in our daily life.
Even founders criticized this ad-hoc QED renormalization method forcibly removing "infinity" as "hocus-pocus" and it must be fundamentally changed, admitting fatal flaws inherent in QED virtual particles.
Physics professors turned into "pseudo-political analysts", science-fiction writers to sell only "literature" books, pursuing illusory "theory of everything" with unreal extradimensions, forgetting practical use of real "physics". Because there is No longer "reality" in the current physics.
See this week physics is still useless.
But in fact, the current mainstream science = Einstein relativity and quantum mechanics blatantly defy this basic physical law, meaning their theories are false.
See this week physics is still useless.
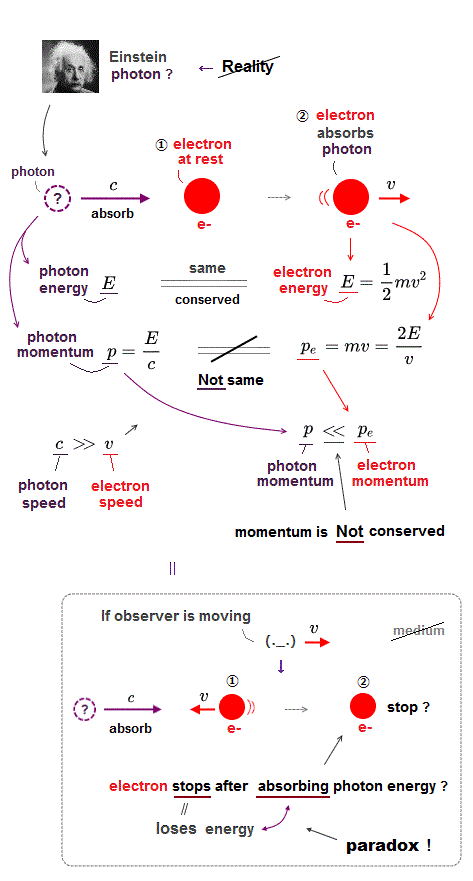
(M-1) ↓ Each electron exists in two atoms in  ?
?
Quantum mechanics cannot treat molecular bonds.
See this week physics is still useless.
(S-1) ↓ Useless physics created unreal concepts = 
The current "science" pursues only illusion such as quantum mechanics, extra-dimensions, God particle, time travel, multiverse, mirror universe..
Physics professors became "pseudo-political analysts". Universities tied with criminal suspects make money by teaching pseudo-science = extra-dimensions.
You may often see incomprehensible word, "symmetry" in various scientific fields from particle to solid physics.
What the heck is this "symmetry" ?
Does this "symmetry" have anything to do with our physical world ?
In fact, this symmetry has nothing to do with real physical phenomena around us, hence, physically meaningless concept.
The present useless physics forced physicists to make up "unreal concepts" called "symmetry" as fictitious scientific goals like fantasy parallel worlds and Big Bang.
Quantum mechanics says each electron can become two conflicting things = a particle and wave, and exist in different places at once like a ghost.
So quantum mechanics can determine nothing about each particle's property (= a particle or wave ) or position. ← Completely useless atomic theory.
In 1920s, quantum mechanics and Einstein relativity were united into one pseudo-theory called "quantum field theory", which is just nonphysical abstract math with No relation to any real objects around us.
Quantum field theory impractically tries to apply only one abstract Einstein mass mc2 equation to every physical phenomenon in the universe in the form of Dirac equation. ← Impossible !
Quantum field theory can only express each electron and photon (= a particle of light ? ) as nonphysical math symbols (= a particle is expressed as a†, b†, c† .. ) without showing any more detailed physical figure of each particle than abstract math symbols.
This mathematical quantum field theory has No ability to explain actual complicated physical phenomena, because it uses only its unrealistic abstract math symbols as fictitious particles.
So physicists had to create other artificial fictitious concepts in order just to develop their pseudo-theory and explain various physical phenomena (= most of which are irrelevant to our daily life ), without showing detailed physical mechanism or delving into underlying reality.
One of these fictitious concepts is an unreal virtual particle as imaginary force carrier with unphysical mass ( this p.3 ).
Other unreal artificial concepts are electron's "spin" which is Not a real "spinning", and "exchange energy" which tries to describe Pauli exclusion principle just by artificial unphysical rule of "particle exchange", which is strangely Not a real force, and No physical interpretation can be given to it ( this p.6 ).
The only way for unrealistic quantum mechanics to explain physical phenomena and develop their pseudo-theory is to increase fictitious particles and concepts meaninglessly, without showing underlying concrete physical reality.
But just increasing unreal concepts or pseudo-particles in a random and disorderly way is purposeless and scientifically valueless, so physicists needed to create some new "artificial rule" seemingly connecting all fictitious particles and concepts to make their pseudo-science look scientifically "meaningful".
This "artificial rule" is so-called symmetry, which was introduced only to develop their unphysical quantum field theory, though "symmetry" has nothing to do with our real world or actual physical phenomena.
"Symmetry" is nothing but an unrealistic, unphysical, abstract math concept or rule fabricated by unreal quantum theory.
Dirac equation invented as the only equation describing quantum field theory is too simple and too abstract to explain various complicated physical phenomena.
Its nonphysical wavefunction (= ψ ) is supposed to represent all fermions such as electrons and quarks. Math symbol "A" represents a "photon". ← No more detailed description of each particle's shape ( this p.20 ), completely useless equation.
Physicists suddenly made up artificial rules = when this nonphysical wavefunction is changed by artificially-introduced "phase transformation" and the whole Dirac equation is invariant, it is called "symmetry" ( this p.11 ).
This "phase transformation" + "invariant equation" = "symmetry" is just an artificial rule which has nothing to do with real physical objects.
By increasing the number of types of these artificial "symmetry transformations", physicists came to increase fictitious particles such as quarks using this ad-hoc rule (= symmetry ) as an excuse to rely on fictitious particles.
When this artificial symmetry is broken by additional mass (= m ) term, this is called "Higgs mechanism", all of these particles are just nonphysical math symbols ( this p.7 ) without physical figure, hence they have nothing to do with our real world.
When this artificial "symmetry transformation" exchanges a particle for its imaginary "shadow particle", it is called "supersymmetry" which unphysical math concept also has nothing to do with our real world ( this p.11 ).
This unphysical concept called supersymmetry is necessary to reduce 26-dimensional string to 10-dimensional superstring theory ( this p.15 ).
Anyway, this string theory a.k.a "theory of everything" is untestable, hence, meaningless theory, because its extradimension can never been seen, and string theory including as many as 10500 freely adjustable parameters cannot predict any physical values.
This is why the leading string theorist, Witten gave up practical use of his pseudo-science (= extra-dimensions ) and is obsessed only with "politics" irrelevant to science now.
Supersymmetry also includes more than 100 freely-adjustable parameters which is completely useless, cannot predict any physical values.
The "reality" of a theory depends on whether the theory actually "influences" and contributes to actual technology improving our daily life.
Just because the theory got prestigious Nobel prize does Not mean the theory is real, as long as it is useless except as "political tool" to waste taxpayers' money in the name of "science".
Actually Nobody uses unseen extra-dimensions, supersymmetric particles, quarks, God particle Higgs, black hole or quantum computer in our daily life. It means all these particles are just worthless fiction, so is the "symmetry".
Physicists think only about wasting taxpayers' money in meaningless gigantic collider or fabricating imaginary particles in order just to develop their pseudo-theory and sell "sci-fi" books, forgetting practical use of real "science".
Nobody around you uses unreal "virtual particles of QED which is useless", because it lacks physical reality from the beginning, and its calculation results always diverge to infinity.
Physicists invented artificial trick called renormalization which cannot predict any physical values like freely-adjustable parameters by artificially subtracting arbitrary infinity from infinity to get finite values, which meaningless dirty trick was harshly criticized even by founders.
Unless we replace the current pseudo-physics by other real practical atomic theories, applying basic physics (← this must be a real theory, first ! ) to curing deadly diseases is impossible forever.
Sadly, also in the current outdated educational system, "going to schools perfunctorily or subsidizing old-fashioned academia" sacrificing students' future by forcing their fake science is its only (fictitious) goal like this unphysical "symmetry", forgetting teaching "real science".
See this week physics is still useless.
(S-1) ↓ Unreal artificial 'exchange energy' created by 

Quantum mechanical electron spin lacks reality.
See this week physics is still useless.
(V-1) ↓ 200-year-old vaccine is the only hope of  ?
?
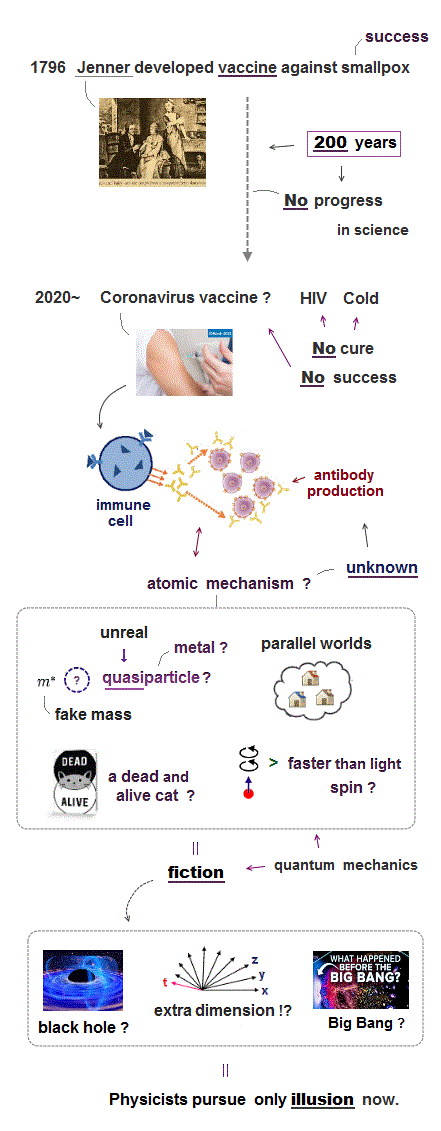
Now all countries and drugmakers are rushing to develop dreamlike coronavirus vaccine as the only hope for containing unprecedented pandemic.
But vaccine development usually takes an incredibly long time.
Even the mumps vaccine, which was the fastest ever approved, took four years.
No vaccines against the coronavirus family such as SARS and MERS succeeded so far. Rapid loss of COVID-19 antibodies indicates both herd immunity and vaccine development will be difficult.
In fact, basic technology of developing vaccine has Not progressed since Jenner first succeeded in using vaccine against smallpox more than 200 years ago.
Vaccine is very simple = inject virus antigen into body → some natural immune cells may produce antibodies against viruses. This traditional old procedure has remained unchanged for more than 200 years.
Antibody test and immunotherapy also use the same old technique of injecting antigens of viruses or cancer cells into animals, expecting their natural immune cells will produce effective antibodies. ← But in most cases, it failed.
The current human technology cannot design or produce antibodies from scratch directly. All we can do is blindly inject some (virus) antigens or proteins into bodies. It is impossible for us to predict how immune cells respond to the injection.
Because precise atomic mechanism of human immune cells producing antobodies is still unknown and uncontrollable.
This is why 'unpredictable' vaccine development takes an extremely long time and may cause unexpected severe side effects.
And almost all vaccine development ends up in failure, wasting huge taxpayers' money.
Even the simplest common cold and HIV viruses are still incurable, despite a long-time research for finding "miracle drug or vaccine".
All problems boil down to the basic science = quantum mechanics that has remained unrealistic, unchanged and stopped all applied science such as medicine from advancing for 100 years.
Quantum mechanics is filled with "physically-contradictory" concepts such as "a particle in two places at once", rewriting the past, afterlife, negative mass, negative kinetic energy, a dead and alive cat, faster-than-light entanglement.
It's impossible to utilize "physically-contradictory" basic quantum mechanics for developing actual science such as medical technology, because "physically-contradictory" means quantum mechanics lacks reality.
Physicists turned this useless physics into a mere political tool (= extra-dimensionist ) and literature (= dark-matterist ) to sell books (= parallel-world believer ), because their pseudo-science has No practical application.
Quantum mechanics was created as the only atomic theory a long, long time ago when physicists couldn't use convenient modern computers. Computers are necessary to calculate many-body effects in many-electron atoms and molecules.
Without computers, it was impossible for physicists to establish a new atomic theory which can handle many electrons and many atoms in solids and molecules.
So quantum mechanics is intrinsically an "impractical atomic theory" which cannot deal with complicated chemical or biological reactions involving many atoms and electrons.
The practical atomic theory has to deal with complicated many-electron atoms. But old quantum mechanics has No ability to deal with many-electron atoms due to lack of modern computers when quantum mechanics was formulated in 1920s, which caused many self-contradictions and unrealistic concepts in today's quantum physics.
Without computers, physicists had No choice but to replace realistic classical atomic model by unreal, unphysical electron's spin model which is coincidentally supposed to generate the same magnetic field (= Bohr magneton ) and energy level difference as Bohr's classical orbit.
But for a very tiny electron to generate the observed strong magnetic field, each electron has to spin far faster than light unrealistically. So physicists made an excuse that electron "spin" does Not mean actual "spinning".
Quantum mechanics started to accept self-contradictroty and unrealistic concepts such as "spin" as the result of struggling to apply complicated physical phenomena to unphysical, ad-hoc theory forcibly in the unmodernized period 100 years ago ( this p.5 ).
Basically, the magnetic force or energy is negligibly smaller and weaker than other forces such as Coulomb electric force or energy.
The magnetic force of electron spin is Not powerful enough to cause actual chemical or biological reaction, because its magnetic force is far weaker than Coulomb electric force, which is the main generator of physical phenomena.
But for nonphysical ad-hoc quantum mechanical theory, this unreal electron spin was the only tool available for researchers to develop their theory and explain complicated physical phenomena in old 1920s.
Because abstract unphysical spin can be simply expressed just as two kinds of arrows or math symbols without showing any concrete physical figure ( this p.2 ), which simple spin description doesn't require sophisticated modern computers.
Of course, it's impossible to describe actual complicated physical and biological reactions using only this simple, unphysical electron "spins" with magnetic force which is too weak to explain influential atomic behavior.
So physicists needed to make up other artificial new concepts called "exchange energy" where Pauli exclusion force is allegedly caused by nonphysical "flipped sign" by exchanging two electrons. ← No more detailed mechanism of how this strange "exchange energy" is generated.
This artificial and unrealistic "exchange force or energy" lacks force carriers, hence any realistic explanation of this strange Pauli exchange force or energy is impossible ( this p.6 ).
Magnetic field utilized in solids such as ferromagnet is supposed to be caused by this unreal electron "spin" with No further detailed physical explanation, except naming it "spintronics".
But as I said, the magnetic force caused by electron spin is too weak to generate meaningful physical phenomena such as the ferromagnetism ( this p.6 ). So the convenient, artificial "exchange force" (= lack physical interpretation ! ) was made up as ad-hoc imaginary savior for explaining ferromagnetic phenomenon. ← too good to be true ( this p.20 )
Quantum mechanics could Not explain mulit-electron atoms.
→ Unreal "spin" was made up in 1920s.
→ This electron spin is Not "real spinning", and its spin magnetic force is too weak to cause actual phenomena such as Pauli exclusion force and ferromagnet.
→ Another artificial, nonphysical concept ="exchange energy" was made up as an ad-hoc savior to help electron spin seemingly explain complicated physical phenomena, sacrificing its realistic interpretation.
→ All physical phenomena such as solid-physics have to be explained by unreal, artificial concepts in quantum mechanics. ← vicious cycle.
So the current condensed matter physics has to rely on unreal quasiparticle model and bogus one-pseudo-electron DFT calculation method, which unphysical quantum mechanical concepts stop our "science" from progressing now. → No dreamlike vaccine or drug can be developed.
Physicists hate "ambiguous" situation. They always crave "some answer", formulation, reason or common physical principle, even if these principles are unreal, artificial and unrelated to our real world.
So physicists were Not allowed to halt developing their ad-hoc theory or hypothesis, just because modern computers necessary to calculate actual many-body physical phenomena were unavailable in 1920s. → Contradictory quantum mechanics had to be made up.
Unphysical theory needed to create other unphysical theories to proceed.
Therefore, physicists made up another artificial, unphysical rule called "symmetry" only to develop their ad-hoc theory, which "symmetry" has nothing to do with our real world.
Under this artificial principle called symmetry, particle physicists started to claim many (fictional) particles might be created with the help of imaginary virtual particles only inside artificial circumstances such as particle colliders.
These unstable, unseen elementary particles are just illusion, as shown in the fact that the only purpose of the current miserable particle physics is to get taxpayers' money for meaningless gigantic colliders, while many people are suffering from unprecedented economic recession by pandemic.
And all other physicists also had No choice but to escape from reality, and seek "imaginary scientific targets" such as fictional parallel-world quantum computer, black hole, BigBang, multiverse, unseen dimensions as "theory of everything" in vain.
These fantasy basic physics is the reason why the current medical technology stalls and old-fashioned vaccine is still the only hope for tackling viruses. ← Strong evidence that science makes No progress recently.
Quantum mechanics introduced in 1920s when there were No modern computers to calculate complicated many-electron atoms or molecules, so quantum mechanics is intrinsically unable to deal with many-body effect or many-electron atoms, molecules, proteins. ← Drug development is intrinsically impossible by quantum mechanics.
So physicists needed to make up artificial circumstances and concepts such as unrealistic "spin" and quasiparticle to seemingly handle many-electron atoms or molecules which are intrinsically impossible to handle by quantum mechanics.
But once even this unrealistic quantum mechanics was accepted as the only ( ad-hoc ) atomic theory and got prestigious Nobel prizes, academia desperately tries to protect these pseudo-science only for sticking to their academic status, even by spreading deadly viruses, endangering people's lives in "dictatorial school" system.
So the first thing for us to do for containing deadly viruses is replace old useless quantum mechanical atomic model by new real one that can treat many-electron atoms using realistic particles with No self-contradiction or unreal spin, which calculation needs computers.
This practical approach to apply basic physics to drug development was impossible for old quantum mechanical model, which was introduced 100 years ago and already contaminated with many artificial, irreparable fictitious concepts such as quasiparticles, spin and fake mass model.
This is why Microsoft, Gates foundation = ardent supporter of vaccine, is also promoting the impossible dream of creating unscientific parallel-world quantum computer using fictional quasiparticle which pseudo-basic physics prevents them from clarifying deeper atomic mechnism of virus infection and immune response to produce effective, safe vaccine.
See this week physics is still useless.
See previous version of criticizing top journals.

2020/6/19 updated. Feel free to link to this site.