Home page
Electron spin is useless
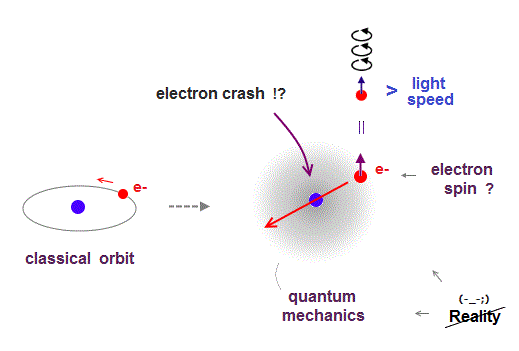
(Fig.1) ↓ A point-like electron must rotate faster-than-light ?

Quantum mechanics claims atoms have unreal zero angular momentum, which means electrons crash into nucleus ! ← nonsense.
They made up an excuse that the electron is a vague "cloud", there is no point thinking about its motion. ← stop pursuing truth !
Quantum mechanics needs an "imaginary spin", which is Not actual spinning, to explain magnetic field of atoms with zero angular momentum.
An electron is very tiny, so its spinning speed must exceed light speed to generate its magnetic field ( this p.2 ), which lacks reality.
So quantum mechanics is unrealistic from the beginning.
(Fig.2) ↓ Classical orbit and electron spin have the same magnet !?
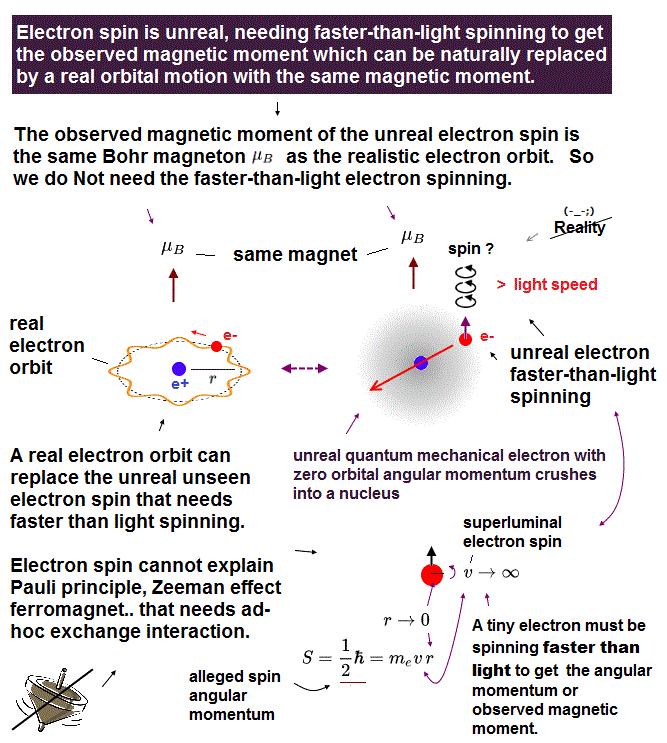
Surprisingly, an electron spin has the same magnet (= Bohr magneton ) as the old classical orbit, using "artificial trick".
They say this electron spin cannot stop or slow down, no matter how many times the electron goes through different reactions. ← unbelievable !
Strangely, spinning electron cannot return to its initial state by 360o degree rotation ! It needs 720o rotation to return !? ← fantasy.
So an electron spin is illusion caused by unreal quantum mechanics.
We can measure only magnetic fields ( Not spin itself ) in experiments, its magnetic field is caused by classical orbital motion instead of unreal spin.
(Fig.3) ↓ Pauli repulsion = 30 eV vs. Spin energy = 0.0001 eV
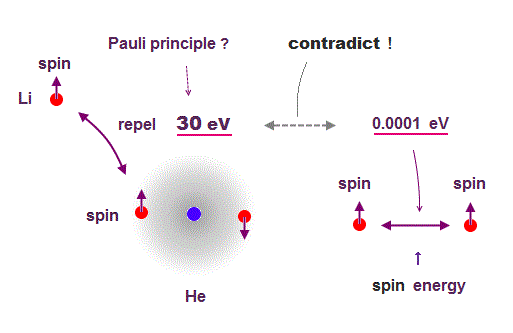
Quantum wavefunction only shows vague electron cloud, which tells us nothing, so they had no choice but to use unreal spin to explain Pauli exclusion principle.
If there is only Coulomb force, the 3rd electron of lithium can enter innermost orbital. But that electron is repelled to outer orbital, which repulsive energy is huge = about 30 eV.
We can caluculate spin-spin energy using its spin magnetic moment, which is very weak = only 0.0001 eV ( this p.6, this p.17 ).
So spin-spin magnetic energy is too weak to cause Pauli exclusion force. ← Spin has nothing to do with Pauli principle !
So they introduced new artificial "exchange force" by which two same orbitals must have the opposite spins, otherwise their wavefunctions are cancelled out to be zero for No detailed reason.
This Pauli "exchange" force is Not a physical force, but just artificial nonphysical concept to explain phenomena.
In this way, physicists have given up pursuing true physical picture about "spin".
(Fig.4) ↓ Average electron spin-up-up magnetic energy becomes zero, so electron spin is physically meaningless.
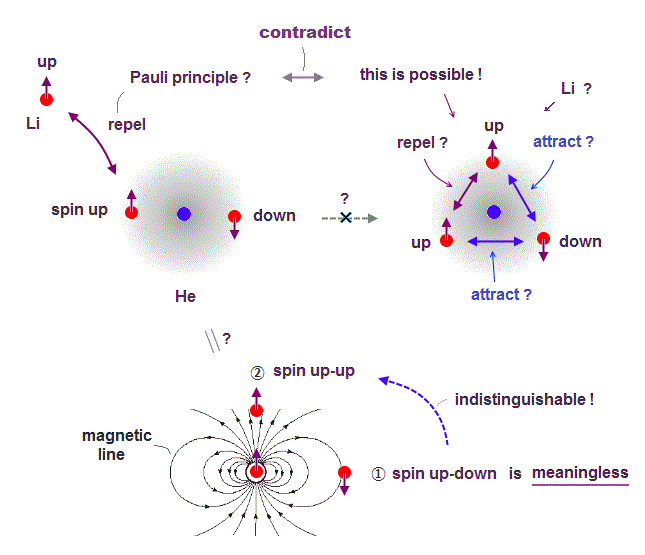
If Pauli principle is true, it causes serious paradox.
If the same spins repel earch other in Pauli principle, it means the opposite spins attract each other.
If so, when one more "spin" enters the same orbital, its total force is attraction (= two opposite spins and one same spins ), so stable, as seen in Fig.4 right.
Each electron has magnetic field. In the position ①, spin "up-down" is stable, because spin is parallel to magnetic field line, so in the position ②, spin "up-up" is stable, along the magnetic line direction.
Considering magnetic field direction caused by spin, both states of "spin up-down" and "spin up-up" are stable and allowed in the same orbital.
↑ It means the magnetic energy between two electrons with spin-up-up becomes attractive and repulsive depending on places, and the average electron spin-up-up magnetic energy becomes zero in all places. ← Electron spin has nothing to do with Pauli principle.
So quantum mechanical claim that "spin up-down" in the same orbital is meaningless to explain Pauli principle.
Spin is unreal, and too weak to cause Pauli principle.
de Broglie wave interference can explain Pauli principle naturally.
(Fig.5) ↓ Pauli principle prediction by spin is false.
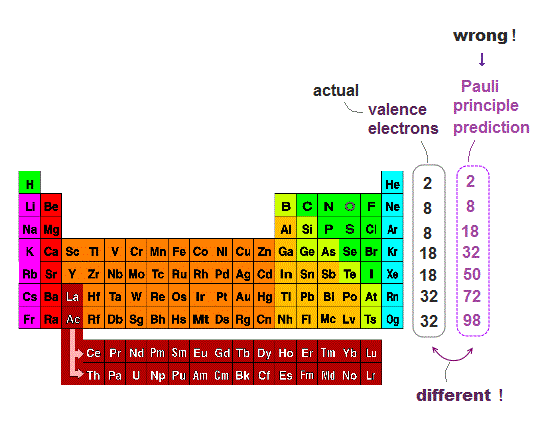
Pauli principle says each orbital can have a pair of spin up and down electrons. So the maximum valence electron number in each energy level n should be 2n2.
This Pauli principle prediction disagrees with the actual valence electron number.
For example, Ar ( n=3 ) has 8 valence electrons, but according to Pauli principle by spin, it should be 2×32 = 18 !
So electron spin not only disagrees with Pauli energy, but also disagrees with valence electron number.
To cover this contradiction, quantum mechanics artificially changes the orders of orbitals ( ex. 3d is later than 4s ) ← nonsense.
(Fig.6) ↓ Unreal spin = fake mark.
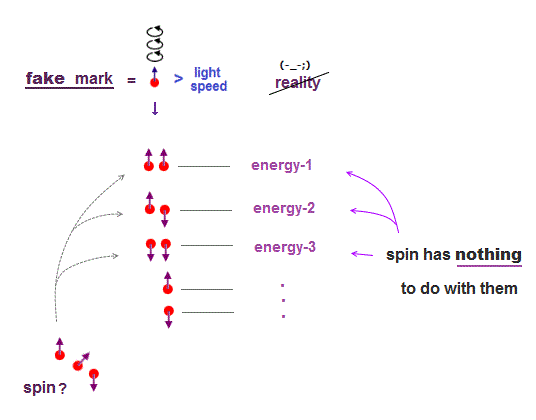
In 1920s when quantum mechanics was born, there were No computers.
To distinguish energy levels caused by multi-electron Coulomb interaction, physicists without computer needed other stuffs, which happened to be fictitious "spin".
Electron spin (magnet) is too weak to cause energy difference among excited states.
Spin is used just as "fake mark" to distinguish atomic energy levels, though unreal spin has nothing to do with them.
(Fig.7) ↓ This was really a lucky coincidence ?
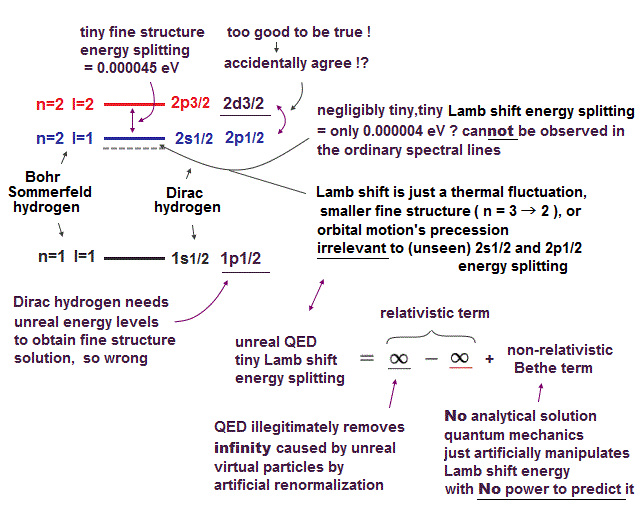
The important point is that the fine structure (= small energy splitting ) of hydrogen was first obtained by Sommerfeld using Bohr's orbit. ← Textbooks hide it !
Later, Dirac equation using spin-orbit interaction got exactly the same solutions as Bohr-Sommerfeld model ! Lucky coincidence ?
It is surprising that Bohr-Sommerfeld model with No spin gives the same fine structure solution as quantum theory.
Clearly, one of them (= latter Dirac hydrogen ) tried to aim at the same solution as the former Bohr-Sommerfeld, using some trick.
Compare this p.12 and this p.1
Einstein relativity contradicts de Broglie wave, so increased mass in Bohr-Sommerfeld model is due to increased resistance from field around an electron, Not by relativity.
Because in Einstein relativity, all concepts such as time, mass, energy are illusion which cannot be utilized as "actual energy", because they increase or dicrease depending on different observers.
(Fig.8) ↓ Sodium (= Na ) is too big spin-orbit effect ? ← unreal.
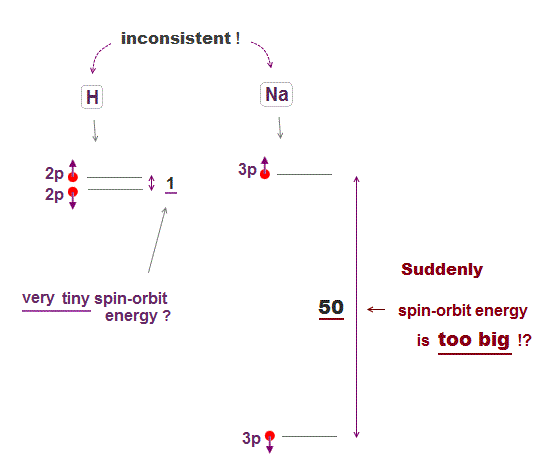
Hydrogen small energy splitting is said to be caused by spin-orbit interaction, which accidentally agrees with Bohr-Sommerfeld model without spin.
The problem is spin-orbit energy difference between hydrogen and Alkali atoms such as sodium is too big, which means spin-orbit is unreal.
From the viewpoint of an electron, a nucleus is rotating around a electron, and it causes magnetic field at the point of electron spin, which causes spin-orbit interaction.
Hydrogen spin-orbit energy is very small (= 0.000045 eV ). If spin-orbit is real, other Alkali atoms' spin-orbit energy should be as small as as hydrogen.
But sodium spin-orbit energy is about 50 times larger than hydrogen.
To generate this unrealistically large sodium spin-orbit energy, the charge of singly-ionized sodium (= Na+ ) must be as large as +3.5e instead of +1e ! ← this is unreal.
Other Alkali atoms' spin-orbit energies are much more unrealistically large.
These large spin-orbit energy splitting is caused by Coulomb energy difference between different orbits like Bohr Sommerfeld model, Not by spin-orbit interaction of the same orbital in quantum mechanics.
(Fig.9) ↓ Energy difference between singlet and triplet is far bigger than spin !
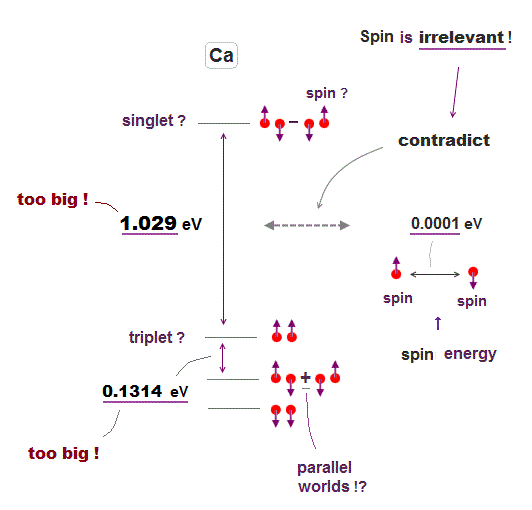
Energy levels in atoms with two-electron valence electrons are called triplet or singlet, which are said to be caused by unreal spin in parallel worlds.
But in fact, these energy levels have nothing to do with spin, because spin-spin magnetic energy is too weak (= 0.0001 eV, this p.4 ).
Energy difference between singlet and triplet states is 10000 times bigger than spin-spin energy, which means unreal spin is irrelevant to atomic energy levels.
For example, energy difference between singlet (= 4s4p, 1po ) and triplet (= 4s4p, 3po ) in Ca is too big = about 1.03 eV, using energy unit converter ( cm-1 → eV ).
Even energy difference among triplet states is 1000 times bigger than spin-spin energy. ← Spin is irrelevant ! This is due to Coulomb energy.
To cover this contradiction, they invented artificial nonphysical concept = (anti)-symmetric wave function, wich has No evidence, because Schrödinger equation is useless in multi-electron atoms.
They say this wavefunction difference between singlet and triplet causes Coulomb repulsive energy difference among electrons, which is far bigger than spin energy ( this p.8 ).
It means "spin" has nothing to do with atomic energy levels, only Coulomb energy does.
(Fig.10) ↓ Spin orders in triplet are inconsistent among atoms !
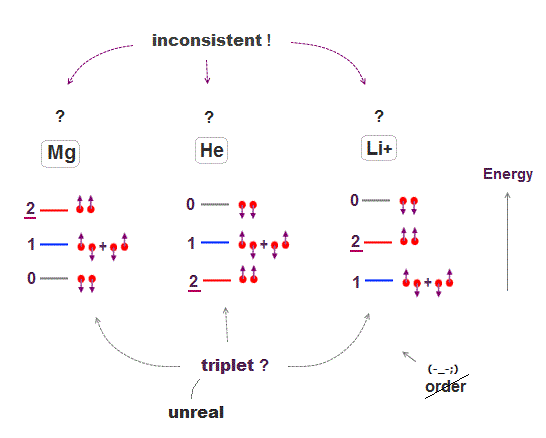
In fact, spin-related energy levels are self-contradictory, so wrong.
Spin's orders in triplet energy levels are inconsistent among atoms ( compare Mg, He, Li+ ), which means there are No common rules governing disorderly spins.
They say each triplet state splits into three energy levels depending on total angular momentum ( J = 0, 1, 2 ).
If there was some common physical principle, these orders must be the same among different atoms, but different ! ← No common principle.
This order is the opposite between Helium and Magnesium.
So "spin" is just "fake mark" introduced temporarily in old 1920s when there were no computers..
(Fig.11) Beryllium (= B ) and ionozed Boron (= B+ ) have the same electrons.
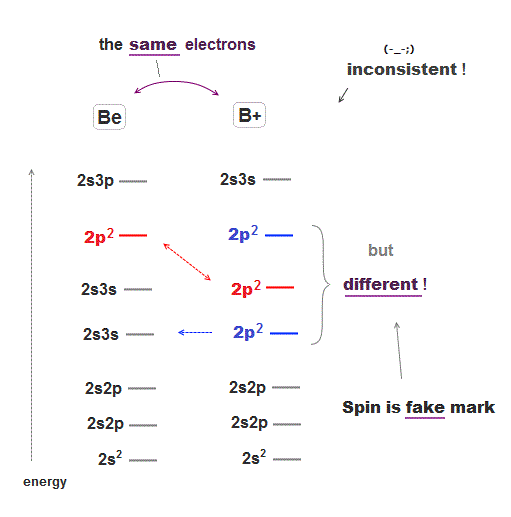
There are many cases which show quantum spin is just "artificial mark" irrelevant to actual energy levels.
Beryllium (= Be ) and singly-ionized boron (= B+ ) have exactly the same four electrons, so their energy levels' order must be the same.
But these two atoms with the same electron structure have completely different orders of energy levels where 2p2 in B+ is lower than 2s3s, which is the opposite to Be.
Mg and Al+ have the same electrons, but their energy levels' orders are different.
As seen in these atomic data, there are No common rules governing energy levels in quantum mechanics based on unreal spin.
So, spin has No experimental evidence at all.
(Fig.12) ↓ Singlet, triplet states are independent, separated !?
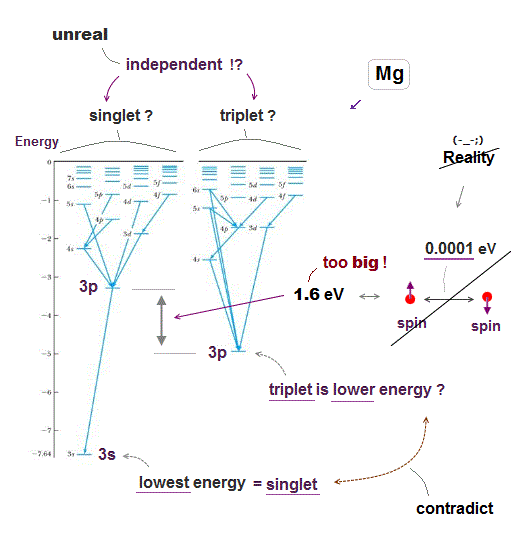
Quantum energy states, singlet, triplet are very unrealistic.
Excited energy levels = singlet and triplet states are separated and independent from each other, there seems to be No communication between these two states.
If transition to triplet state is forbidden or very slow, it cannot explain very strong energy transiton lines among excited triplet states ( this p.18 ).
"Separated" singlet and triplet energy diagram shows these energy levels by quantum mechanical interpretation is unreal and artificial.
As seen in Fig.12, when they artificially try to put singlet and triplet of higher levels (= 5d, 6s ) at the same location, lower levels (= 3p ) have to cause wide discrepancy between singlet and triplet, which is too big for spin energy.
They say triplet state is lower than singlet state, because Coulomb repulsion between electrons becomes weaker by cancelling their antisymmetric triplet wavefunction.
But this explanation contradicts the fact the lowest energy (= ground ) state is singlet, so wrong. Anyway, useless Schrödinger equation cannot estimate them.
They forget the fact that cencelling wavefunction in triplet means attractive energy between electron and nucleus is cancelled, too, which influence is bigger than electrons' repulsion, so singlet should be lower than triplet in energy level.
Quantum mechanical interpretation of "triplet lower than singlet" is nonsense and wrong.
(Fig.13) ↓ Quantum solid physics uses "fake electron" with fake mass
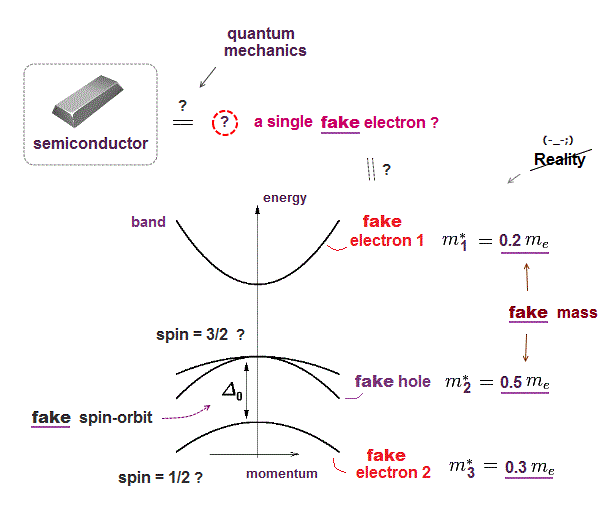
Quantum mechanics is useless in multi-electron atoms, so they had no choice but to treat the whole solid as if it were a fake "one-electron" in band theory ( this p.3 ).
This fake electron has fake (= effective ) mass, which mass can be negative, and artificially changes in different directions ( this p.2 ). ← unreal !
Quantum mechanics uses this fake electron with fake mass to derive fake spin-orbit interaction ( this p.5 ).
This spin-orbit interaction in solid physics is unreal, contradicts Einstein relativity.
Because they artificially changed the original definition of spin-orbit interaction, so that it is far stronger than that of Einstein relativity (= vacuum ), and has the opposite sign ( this p.24, this p.2 left, this p.9 ).
Ab-initio method in the current solid physics is just to compute these fake effective mass using density functional theory (= DFT ) with unreal pseudo-potential.
So quantum mechanics relies on fictitious concepts in solid physics, which is useless forever.
(Fig.14) ↓ Spin in quantum mechanics is fake !
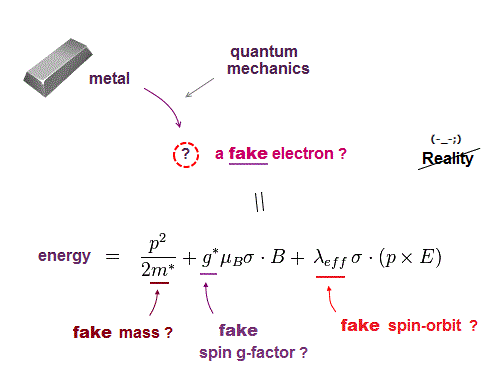
Quantum mechanics cannot handle multi-electron atoms, so they had no choice but to use fake electron with fake (= effective ) mass.
This means all physical parameters such as spin-orbit, spin g-factor, electron mass in quantum mechanics are fake, unreal ( this p.2-8, this p.24 ).
They invented many artificial models in band theory, and change the definition of spin-orbit, so that it contradicts Einstein original relativity ( this p.7, this p.7, this p.2 ).
As you see, quantum "spin" is unreal, it has No physical evidence.
(Fig.15) ↓ A electron splits into "charge" and "spin" (= spinon ) !?
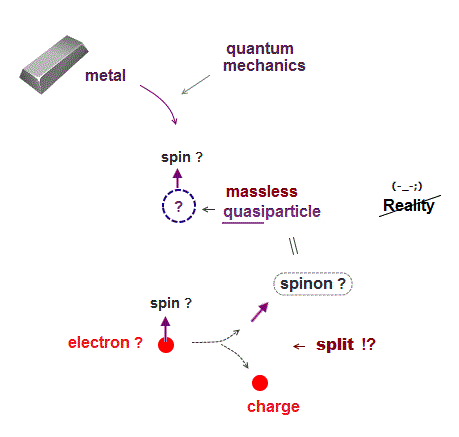
In 1920s when quantum mechanics was born, there were no computers.
So, quantum mechanis had to rely on fictitious concepts to describe atomic behavior instead of computers.
Quantum mechanics uses fictitious unreal quasiparticle with adjustable fake mass and charge to explain physical macroscopic phenomena.
They introduced fake quasiparticle with zero fake mass and fake spin.
And they believe illusion that a single electron can split into "charge" and quasiparticle spinon (= spin ) inside solid ( this p.6-7 ) !
As shown here, "spin" is fake concept, with No physical evidence.
(Fig.16) ↓ This experiment just measured magnetism ( Not spin ! )
Faster-than-light electron spinning is unreal, which cannot be observed directly.
All we can measure is magnetic field of spin, which accidentally agrees with Bohr magneton like fine structure.
Electron spin disagrees with various experiments. Spin-spin interaction is too weak to explain ferromagnetism and Pauli principle, so spin is unreal.
Electron spin is too weak to explain alkali fine structure, triplet, spin-orbit. Spin is just fake marks to distinguish energy levels.
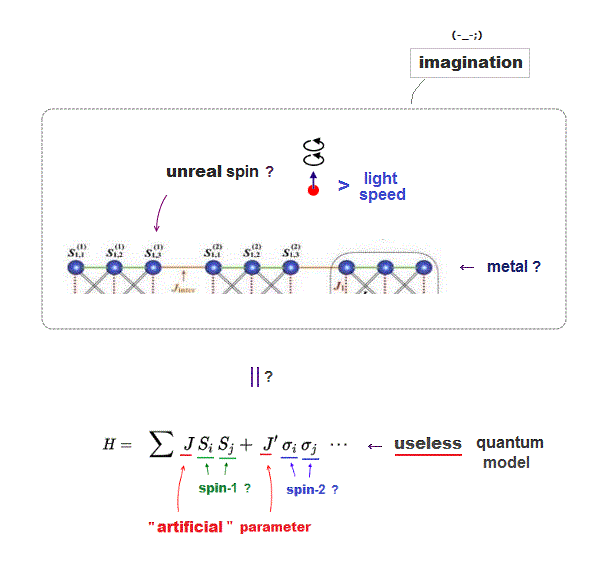
Quantum spin model is too abstract to describe actual phenomena, they just artificially adjust parameters (= J ) depending on experiments ( this p.2, this p.3 ).
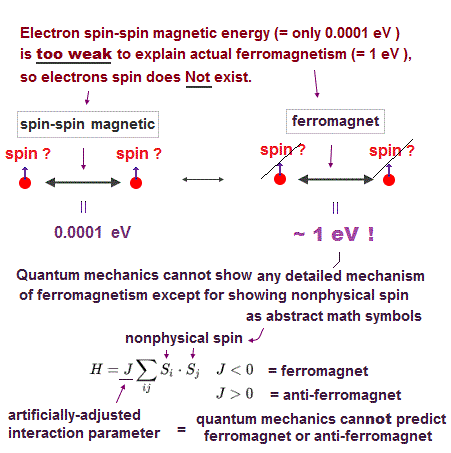
(Fig.17) Spin magnet is too weak to explain ferromagnet.
Electron spin lacks reality !
Its spinning far exceeds
light speed.
Spin-spin magnetic interaction is too weak to explain actual ferromagnet. See this p.6 this p.7.
Ferromagnetism needs strong interaction between atoms (= electron's orbital motion + Coulomb ) instead of unreal weak spin.
Then, what the heck does this spin model mean ?
It uses old "Heisenberg" spin model ( this p.3 ).
But this Heisenberg spin model is too old, which was introduced in 1920s, and it's too abstract to describe actual phenomena ( this p.2 ).
Electron spin is unreal, too small to explain ferromagnet, and too abstract to be useful.

2018/9/4 updated. Feel free to link to this site.