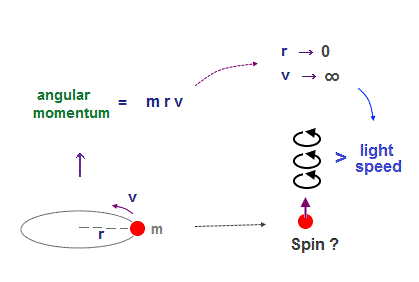
(Fig.1) Point-like electron ( radius r → 0 ), rotation v → ∞

In fact, electron spin is physically impossible, because its spinning speed must far exceed light speed c to reach that angular momentum.
Angular momentum is given by mv × r ( v = velocity, r = radius ).
Electron spin also has angular momentum 1/2ħ, they claim
The problem is an electron is very tiny, point-like.
The point-like particle means its radius r is almost zero.
So to get the angular momentum 1/2ħ, the electron spinning must far exceed light speed ( this p.5, this p.2 )
So the electron spin lacks reality, which unrealistic quantum mechanics fabricated by force to explain experiment.
(Fig.2) ↓ Lucky coincidence ? Spin model is artificial.
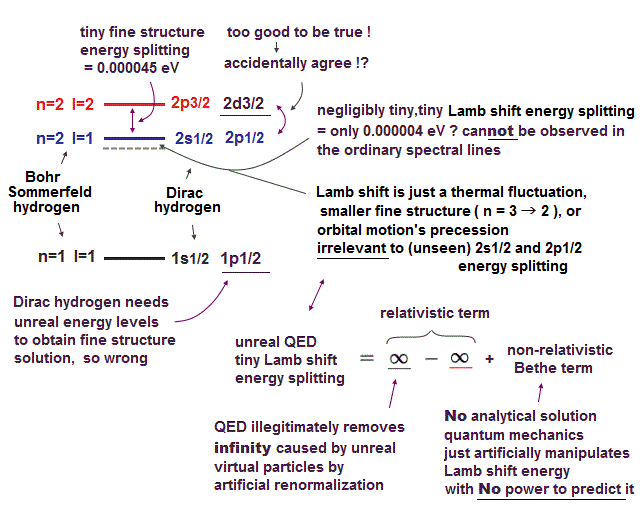
We cannot see electron spinning directly. They just estimated its existence only from atomic emission spectrum in transitions between energy levels.
Typical example of what physicists base spin on is fine structure (= small energy splitting ) of hydrogen.
The point is the fine structure was first obtaied by Bohr-Sommerfeld model with No spin. Later, quantum mechanics using spin immitated it.
This quantum mechanical spin model is based on very unnatural assumptions and false logic.
So various spectral lines such as fine structure didn't prove the existence of "spin".
(Fig.3) Singlet, triplet need parallel worlds of spin !
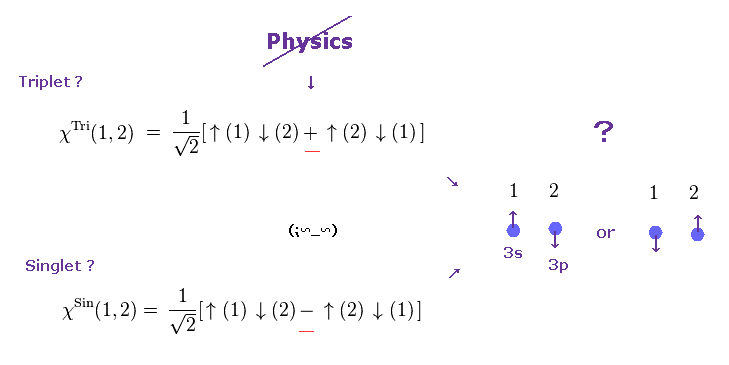
It is often said that atomic energy levels of singlet and triplet proved the existence of spin. Of course, they didn't see the spin itself.
They just imagine how electron spin bahaves in the singlet and triplet states. The point is these singlet and triplet consist of fictional parallel worlds.
In both singlet and the middle of triplet states, one spin is up and another spin is down. Despite their same spins, their energy levels are completely different !
Why does such a energy difference happen between the same zero spin states ? They claim singlet and triplet include two different states at the same time.
In singlet, the sign of two spins | ↓↑ > and | ↑↓ > is the opposite, and its sign in triplet is the same.
So spin singlet and triplet states depend on unrealistic Schrodinger's cat which can be dead and alive (= two different states ) at the same time.
So spin model lacks reality from the beginning.
(Fig.4) Nist uses cm-1 instead of eV as energy unit.
Nist uses ( cm-1 ) as energy unit instead of eV.
10000 cm-1 is equal to 1.23 eV using this converter.
(Fig.5) Difference between singlet and triplet is too wide for weak spin.

Though most textbooks often say atomic spectrum lines of singlet, triplet are related to spin, this is physically impossible.
It is known that the energy difference between the singlet and triplet is so large that weak spin-spin magnetic interaction can never explain it.
For example, in singly ionized lithium (= Li+ ), the energy difference between singlet (= 1S ) and triplet (= 3S ) in 1s2s is very big, 1.90 eV.
On the other hand, magnetic energy based on electron spin magnet is far smaller (= 0.0001 eV, see this p.4 ).
So it's impossible that very weak spin-spin interaction causes very bigg singlet and triplet difference. These are caused by Coulomb force, not spin.
(Fig.6) Spin-spin energy is too weak to cause singlet, triplet.
Spin-spin magnetic dipole energy is much smaller than the energy difference between singlet and triplet.
So electron spin has nothing to do with singlet, triplet. To solve this, physicists created artificial new force, "exchange force" ( this p.7 ) for Pauli exclusion.
But it's a dirty trick to depend on new artificial rule to solve discrepancy between the original theory (= spin magnet ) and the experiments (= singlet, triplet ) !
The Story of Spin by S.Tomonaga says singlet, triplet are as big as those of electric origin, as shown in this.
(Fig.7) Directions of magnetic field are the opposite in different places.
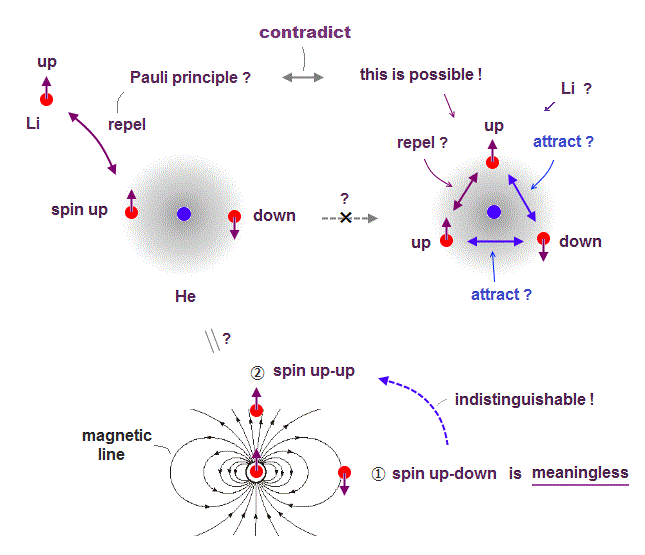
Here we explain why spin-spin magnetic interaction is impossible.
Because the total magnetic energy by spin-spin is always canceled out to be zero.
Magnetic source always includes a pair of N and S poles, which generates magnetic field lines around it.
To retuen from N to S pole, the magnetic field must include two opposite directions ( upward and downward ).
Suppose two spins are always "up" and "up". In Fig.7 left, spin direction is the same as magnetic field caused by another spin, but the opposite in Fig.7 right.
So total magnetic energy in spin-spin always becomes zero, in any spin directions.
(Fig.8) Orbital and orbital magnetic interaction.
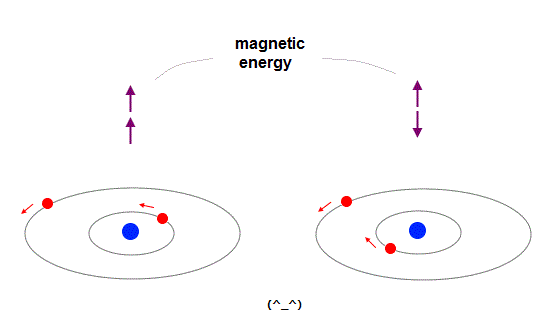
On the other hand, the magnetic energy caused by orbit-orbit intearaction is possible, when one orbit is always inside another outer orbit.
So considering realistic orbit instead of absurd spin is more rational and reasonable from the viewpoint of physics.
(Fig.9) Hyperfine structure between 1s electron spin and nuclear spin ?
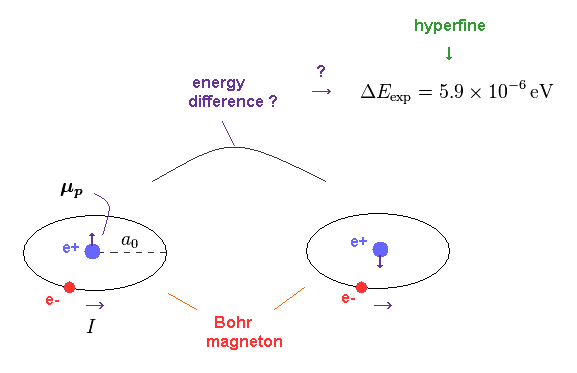
It is said that very weak hyperfine structure is caused by the interaction between 1s electron spin and nuclear spin.
But as I said, spin-spin interaction is always canceled out and becomes zero. So they started to claim hyperfine is caused by 1s electron penetrating the nucleus !
Because the orbital angular momentum of 1s state is zero.
But this quantum mechanical model is impossible and violates energy conservation.
Instead of this irrational quantum mechanics, hyperfine structure can be naturally explained by the interaction between the electron's orbit and nuclear spin.
A proton is much heavier and bigger than an electron, so its spinning doesn't exceed light speed. Actually nuclear magnetic moment is much smaller than an electron spin.
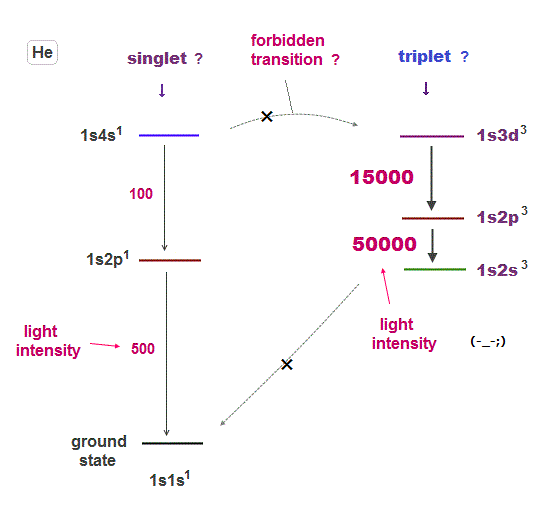
(Fig.10) ↓ Unrealistic separation between singlet and triplet !
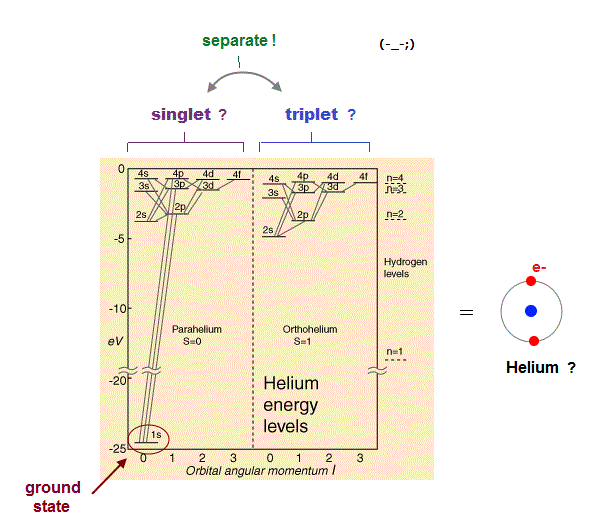
The problem is singlet and triplet states are completely separated from each other, and No communication is seen between them !
This is due to one of artificial selection rules restricting some transitions ( this last, this p.4 ). There is no physical reason for this strange rule.
This rule forbidding transitions between singlet and triplet states is contradictory. Ground state of helium is 1s2 singlet.
If transition between singlet and triplet states are forbidden, why can we see emission spectral lines between triplet and triplet ?
If electron cannot transfer from the ground state (= singlet ) to the excited triplet, even transition light between triplets cannot be seen !
(Fig.11) ↓Why spectral lines between triplet-triplet can be seen ?
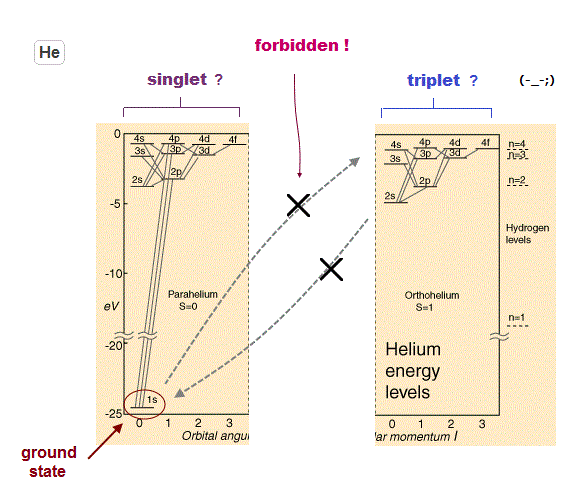
If transitions between singlet and triplet states are forbidden by quantum mechanical rules, this causes serious contradiction.
It means an electron cannot be excited to triplet states from ground state (= singlet ), and there are No triplet states !
So even transition lines between triplet and triplet states couldn't be seen, if this forbidding rule is right.
But strong transition lines among triplet-triplet are actually seen ( in Terms, 3S 3P ). This is a serious self-contradiction.
(Fig.12) Different patterns are allowed as energy levels.
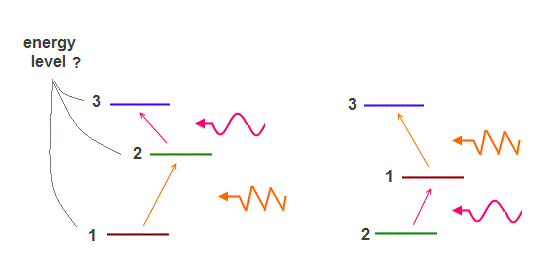
The point is we cannot see each energy level itself. All we can observe is emitted ( or absorption ) lights which are estimated to cause transition between two energy levels.
So all informations we can get is "differences" among estimated energy levels. When we observe two kinds of emitted lights, different energy patterns are allowed.
It means singlet and triplet states based on spin lack direct physical evidence. These are just speculative states.
(Fig.13) Too many transitions are forbidden !
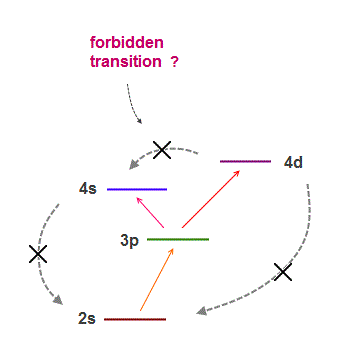
If orbital include electron spin, too many transition lines must be seen.
But transition lines are far fewer than expected by quantum spin model.
So physicists created artificial rules to forbid some transitions, which is called selection rules.
One of them is orbital Laporte rule forbidding transitions between s-s, p-p, d-d and s-d orbitals.
Due to these artificial rules, each energy level can be independent from each other, which means they can easily manipulate and create artificial states.
Though various changes in the total angular momentum ( ΔJ = 0, ± 1 ) are allowed, why the transitions of J = 0 → 0 and p → p are forbidden ? Ridiculous.
(Fig.14) Why "forbidden" triplet can show much stronger spectral lines ?
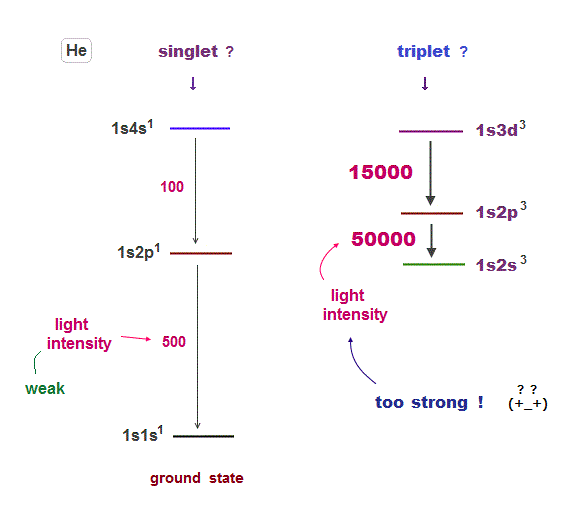
As I said, transitions between singlet and triplet are forbidden by quantum mechanical artificial rules.
In spite of this forbidding rule, emission lights between triplet states are clearly seen, though an electron cannot be excited to triplet states.
So again they created a new artificial concept, intersystem crossing by which singlet electron can transfer to triplet in the "nonradiative process".
Or triplet electron can return to singlet ground state by phosphorescence, which emits very weak and longer-time light ( this p.10 ).
Weaker and non-emitting transitions between singlet and triplet mean the probability that an electron transfers to triplet is extremely small.
But in helium, triplet-triplet light intensity is much stronger (= 30 times ) than those between singlet-singlet ( see Table 1 ) !
(Fig.15) Triplet light intensity is 30 times stronger than singlet. Why ?
Quantum mechanics forbids transitions between singlet and triplet.
Or with extremely low probability, it can transfer to triplet by nonradiative and phosphorescence process.
Forbidden transtion decreases the supply of excited electrons into triplet states, so even transtion light intensity between triplet and triplet should be very weak.
But on the contrary, the light intensities among triplet states are extremely strong, 30 times stronger than singlet light ( this p.26 )
This wide discrepancy between forbidden transition and strong triplet light is clearly unnatural and contradictory.
It is natural to think there is some fundamental mistake in this singlet-triplet rule. ( light intensity in nist sets the maximum intensity at "1000", so different from original data ).
(Fig.16) Very strong triplet should be far closer to ground state.
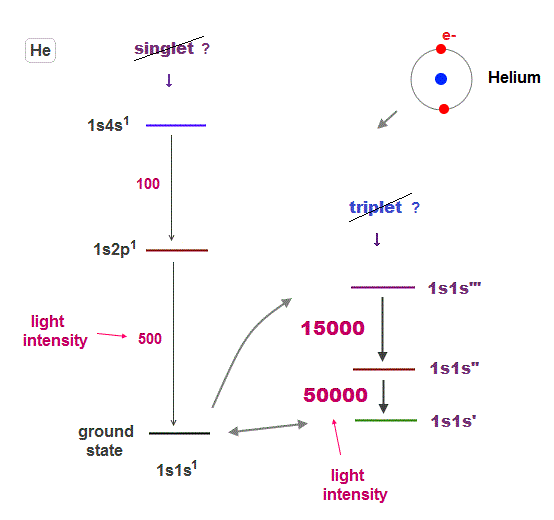
Though transition to triplet from ground state is almost forbidden. triplet light intensity is much stronger than the singlet ! This is clearly a contradiction.
This discrepancy naturally makes us think that very bright triplet states are much closer (= lower ) to the ground state.
The closer to ground state, the stronger the emitted light becomes.
Quantum mechanical singlet-triplet concepts clearly disobey this logic.
(Fig.17) Two electrons approach each other →total energy becomes higher.
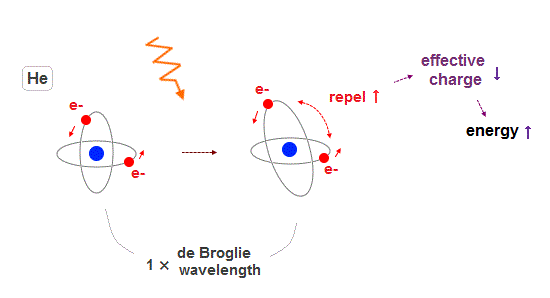
Different from a simple one-electron hydrogen atom, there are two electrons in helium. So the positional relationship between two electrons affects energy.
When two electrons are a little closer to each other, the total energy becomes a little higher due to increased Coulomb repulsion.
In this case, the effective central change which a helium electron feels becomes smaller due to this increased repulsive energy.
In one-electron hydrogen, there are no other electrons which change the "effective central charge" which each electron feels as a nucleus.
It is more natural that excited helium includes various n=1, n=1 states with a little higher energy, rather than crowding only much higher n = 2 as singlet, triplet.
(Fig.18) 3p orbital is higher than 3d in singlet of helium !?
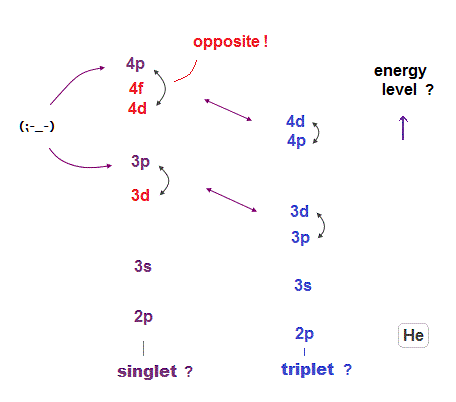
According to quantum mechanics, 3d orbital must be higher than 3p orbital. But helium has higher 3p than 3d only in singlet.
This is clearly inconsistent with the original definition !
In Table.2, singlet 4p is higher than 4d and 4f !
This self-contradiction is caused by artificial selection rule.
And this proves quantum mechanical orbitals have nothing to do with actual energies.
(Fig.19) The relation between spin-state and energy is incoherent.
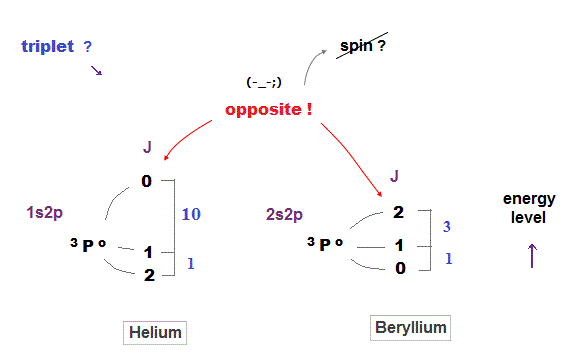
As I said, the large difference between singlet and triplet proves "spin" has nothing to do with singlet, which is caused by strong Coulomb force.
Then how about small energy splitting inside triplet state ?
Unfortunately, even triplet has nothing to do with spin !
Comparing helium and beryllium, you notice the orders of spin states are chaotic.
In helium, triplet state of total angular momentum J = 2 is the lowest, but in beryllium, J = 0 is the lowest. In lithium, J = 2 is the middle !
Energy intervals inside triplets are different and incoherent, too. This result shows spin has nothing to do with triplet energy splitting.
In this book p103, they say, "however in helium 1s2p 3P terms, the order is not only reversed, but the P0 - P1 separation is more than 13 times larger than P1 - P2".
According to Lande rule ( this p.4 ), 3P0 must be the lowest in triplet and separation ratio should be 1 : 2, which is completely inconsistent with helium.
(Fig.20) Beryllium (= Be ) and Boron ion (= B+ ).
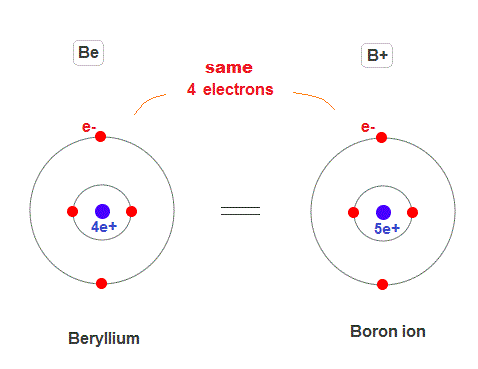
According to periodic table, beryllium (= Be ) has 4 electrons, and boron (= B ) has 5 electrons.
So singly ionized boron (= B+ ) has the same 4 electrons as beryllium. Both Be and B+ has 2 outer electrons like helium.
This means these two atom and ion should show the same kind of energy levels in singlet and triplet states ?
(Fig.21) The order of 2p2 state is chaoric in Be and B+.
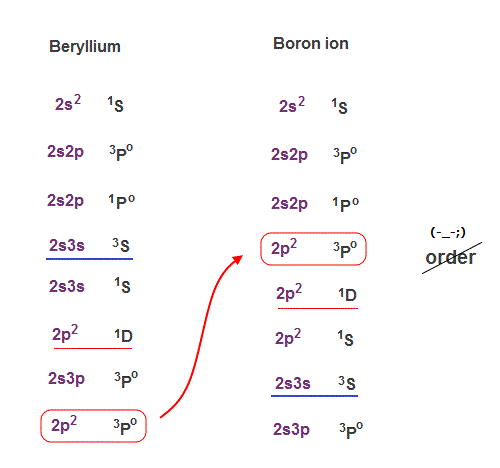
Beryllium (= Be ) and boron ion (= B+ ) have exactly the same electron structure. So they should show the same kind of energy levels in singlet and triplet.
But comparing Be and B+, you notice the orders of their energy levels are chaotic and incoherent !
Triplet 2p2 state of B+ is in much lower energy position than Be.
This data shows there is No consistent law in quantum mechanical triplet.
So these chaotic singlet and triplet cannot be used as a proof of spin, because the energy splitting is irrelevant to quantum mechanical spin model.
(Fig.22) Spin is meaningless mark, Not representing real state.
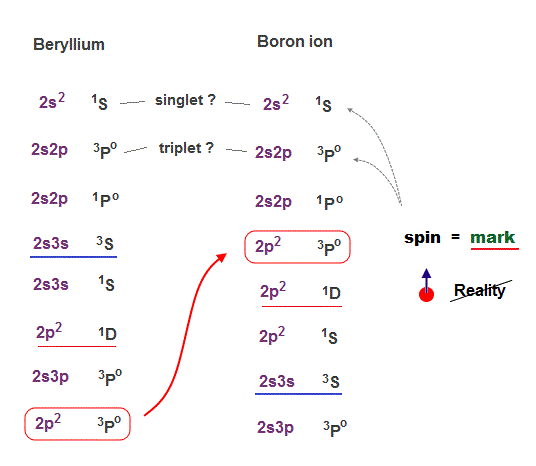
In conclusion, unrealistic "spin" does Not represent real state. Spin is used only as a "mark" of different energy levels.
Because quantum mechanics with No concrete orbit cannot consider realistic states flexibly.
All quantum mechanics can depend on is "orbital and spin" as marks to define each energy level.
This is the reason why unrealistic spin is so often seen in various journals and university's websites, though electron spin has No meaning except as a mark.
(Fig.23) ↓ H atom fine structure is relativistic effect ?
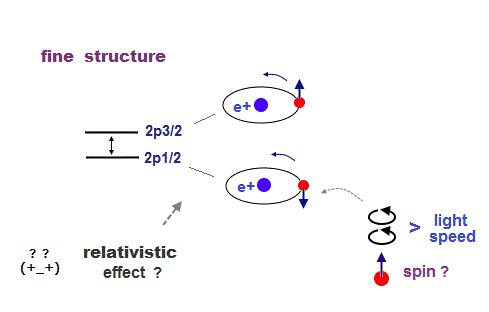
Hydrogen atom has small energy splitting (= fine structure ).
They say this splitting shows the difference between electron's spin up and down.
This spin-orbit interaction is said to be Einstein relativistic effect.
In H atom, an electron is moving around a proton (= nucleus ).
From the electron's point of reference, the proton appears to be moving.
Einstein relativity is based on purely relative ( not absolute ) motion.
So even if the proton is actually stationary, the electron feels the pseudo-magnetic field created by moving proton, which causes small energy splitting depending on spin direction ?
The point is this relativistic electromagnetic fields cause fatal paradox of Lorentz force ! So spin-orbit coupling model is completely false
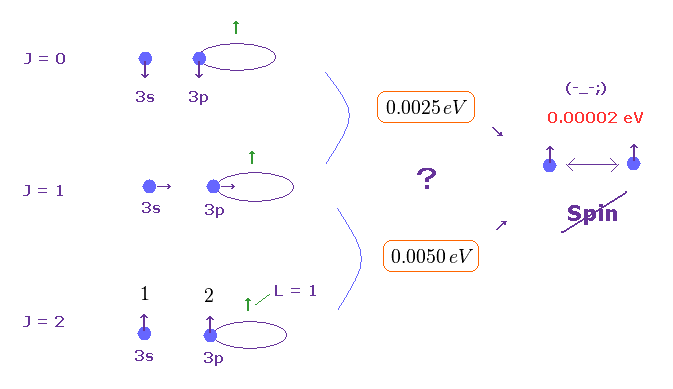
(Fig.24) Na+ ion must have 3.5 positive charge, if spin-orbit is true.
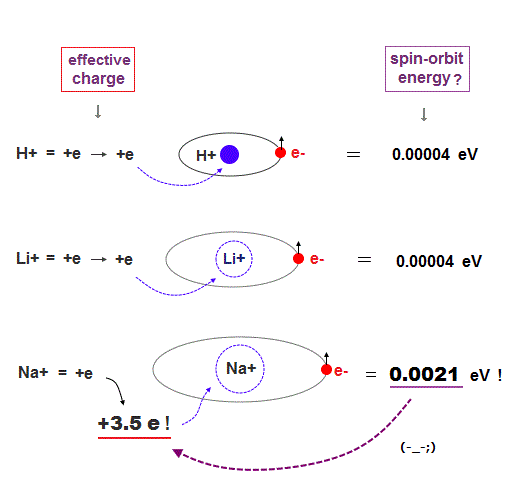
They say Na large fine structure in D lines is also due to spin-orbit interaction. The problem is this Na fine structure splitting is too big.
Compare fine structure splitting in H (= 0.000045 eV ) and Na (= 0.0021 eV ) atoms.
It is known that this fine structure splitting is proportional to Z4/n3, where n is principal quantum number. See this last and this p.4.
Z is effective positive charge (= H+, Na+ ion ), which movement causes magnetic field at the electron's spin ?
To get the large Na fine structure, this central charge (= Na nucleus + all inner electrons ) must be unrealistically big ( Z is +3.5 ).
In other alkali atoms, the situation becomes much worse.
So relativistic "spin-orbit" interaction is too weak to cause alkali fine structure.
Students ( including dropouts ) suffering from debt should sue universities for destroying all students' careers by exorbitant tuition and wrong theories.
(Fig.25) Electron spin is unreal !

So the conclusion is that electron spin has nothing to do with singlet, triplet, doublet (= spin-orbit ? ) spectral lines.
On the contrary, electron spin is inconsistent with fine structure, Pauli exclusion, ferromagnet and singlet-triplet splitting.
Unless we discard this absurd spin, we cannot advance, and our science will stop forever.

2016/2/19 updated. Feel free to link to this site.