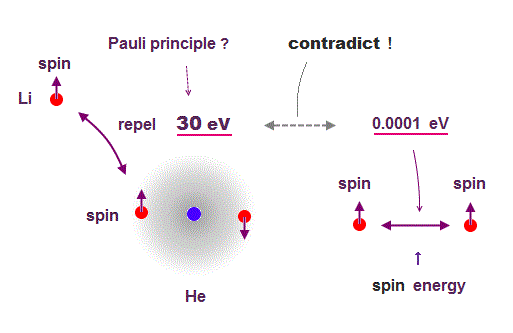
(Fig.1) Spin-spin magnetic energy is very weak ( < 0.0001 eV ) !

Textbooks often say atomic energy levels can be explained by electron spin singlet-triplet expression. But spin energy is too weak to explain actual experimental results !
Experiments showed that each electron ( spin ) has definite magnetic field. We can calculate spin-spin energy using the equation of magnetic-magnetic interaction.
Spin-spin magnetic energy at the atomic radius ( ~ 1 Å ) is extremely weak (= less than 0.0001 eV, this p.6 ) !
(Fig.2) Singlet state is 1.612 eV higher than its triplet in Mg 3s3p energy level.
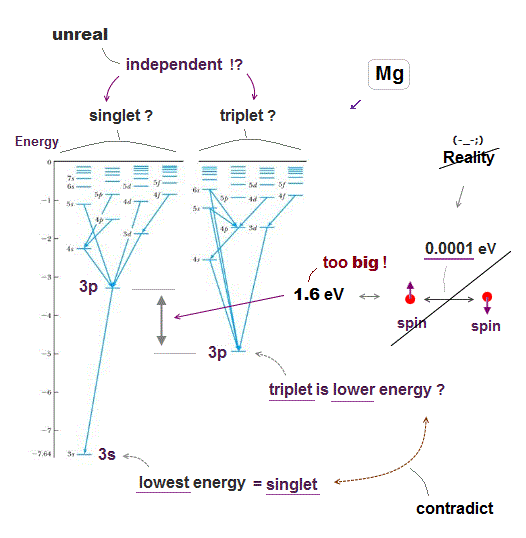
But the actual energy difference between singlet and triplet state is too large to apply weak spin-spin energy in it.
The singlet state is 1.612 eV higher than its triplet state in Magnesium 3s3p ( using energy converter ). But spin-spin magnetic energy is less than 0.0001 eV !
So the singlet-triplet energy levels have nothing to do with unrealistic spin.
(Fig.3) ↓ Mg 3s3p triplet (= 3P ) energy intervals. ← too wide for spin !
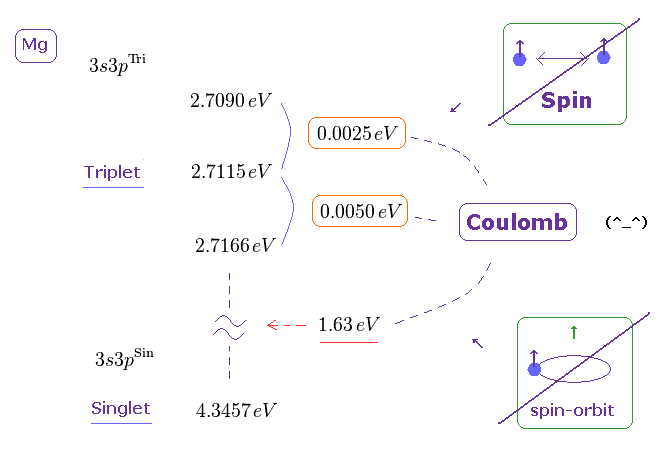
Even triplet energy splitting cannot be explained by weak spin-spin energy.
As shown in this, the energy difference among three Mg 3s3p triplet states (= 3P ) is about 0.00372 eV, which is too large for spin-spin energy ( < 0.0001 eV ).
Spin-orbit interaction energy is also too weak to explain this triplet splitting in metals. So singlet-triplet energy splitting is due to strong Coulomb force, not unrealistic spin !
Furthermore, Schrodinger equation cannot predict each energy levels in multi-electron atoms, so quantum mechanics has nothing to do with all singlet-tiplet and doublet !
(Fig.4) "Spin" is used as "artificial marking" for different energy levels !
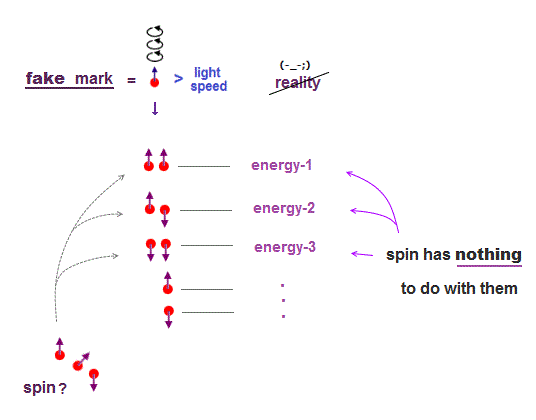
The current basic science has stopped since old quantum mechanics in 1920s. At this time, physicists couldn't describe each energy level by complicated multi-particle Coulomb effect.
This is the reason why they adopted artificial spin as "fictitious mark" to distinguish different energy levels. So spin is not real but just a temporary "tool".
If they couldn't name each different energy level using "fictitious tool of spin", they could have neither published their papers nor proceeded with their academic researches.

2017/1/27 updated. Feel free to link to this site.