Top page (correct Bohr model including the two-electron atoms)
Strange "spin" is NOT a real thing
Why they never tell the truth ?
True hydrogen bonds and steric hindrance (13/6/6).
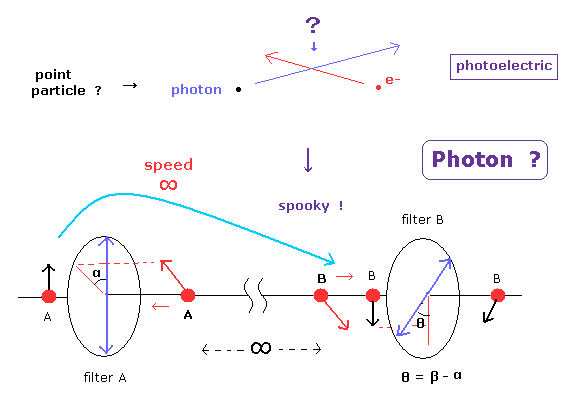
(Fig.1) Photoelectric effect → photon ? → spooky link ! entanglement !

Usual textbooks often explain photoelectric effect showed photon is a particle, not wave.
But as you know, light "frequency" is just same as c / wavelength, so it completely represents wave nature ( NOT particle ).
Like Bohr's electron radiating energy, the present physics textbooks distort the true facts, and brainwash ordinary people into believing wrong facts too much, I think.
As shown in this page, faster-than-ligh spooky link is caused by this illusory concept of "photon".
Of course, Einstein would have never admitted "photon particle", if some entanglement experiments were performed in his time, I think.
(Fig.2) Virtual photon violates special relativity !
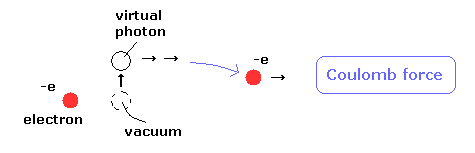
Actually, if you deny ether, and admit "photon", you MUST accept unrealistic virtual photons, which is faster-than-light.
Of course, Einstein NEVER agreed with quantum field theory and QED.
He actually opposed "photon particle" in his true feeling, I think.
According to this book ( The trouble with physics, Lee Smolin ), Einstein had been ignored by physical academic society in his later life, because he never believed quantum mechanics.
An episode with Freeman Dyson was very impressive, in which Dyson was dissapointed at Einstein's unified theories (= NOT using quantum mechanics ), and had decided to avoid him after that.
In spite of his very painful true life, why Einstein have been "deified" in the present physical world ?
(Fig.3) Why and when did Einstein start to be "deified" ?
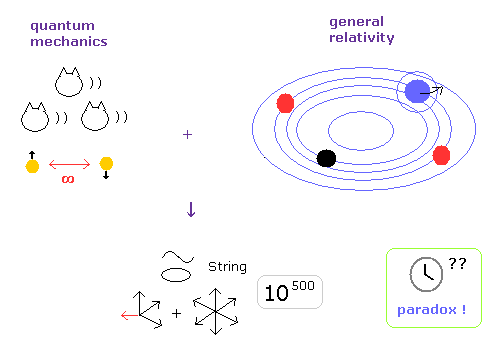
The main reason exits in unrealistic 10-dimensional string theory, which is the only final theory of quantum mechanics and relativity.
They "needed" to make Einstein a charismatic physicist to let ordinary people and students believe unrealistic string theory.
And various media and science writers promoted this trend.
Due to fatal paradoxes of special relativity, rejection of all relativistic theories such as string theory, QED, general relativity and standard model is only a matter of time.
( Probably Einstein himself was suffering from these paradoxes, quantum mechanics and "reality". )
If these serious distorted states dragged on, more new students would be deceived and distorted about his later life.
Can you really take the responsibility of it ?
(Fig.4) General relativity has really "passed" every single test ?
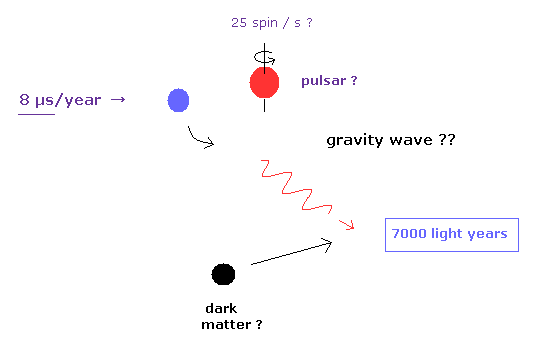
We often see the comments such as "present quantum mechanics and general relativity have passed every single test, and agree with all experimental results ever done." in various web news.
But is it really so ?
For example, in some top journal, we see the results that dwarf star, which is 7000-light-year far away, emits illusory gravitational waves, loses energy, and changes its orbital period slightly (= only 8 microsecond per year ! ).
As you feel, it is impossible that we exclude all other effects such as unknown dark matter, gravitational forces of many other stars.
Even on the earth, we cannot know the detailed history 7000 years ago.
How can you distinguish various infinite noises, unkown dark matter's effects on 7000-light-year signal path ?
It is impossible.
Are researchers doing experiments having "preconceived" notion that general relativty is absolutely right ?
(Fig.5) Fractional charges such as -1/3, +2/3 are real ?
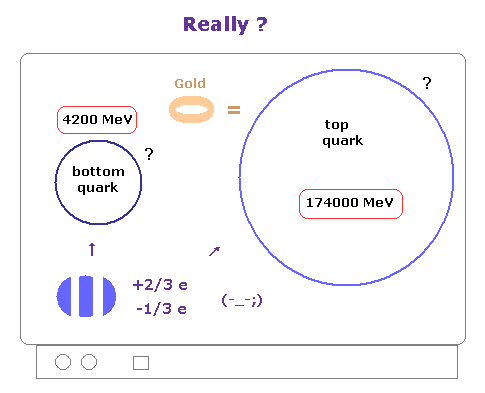
You may often see the comments such as standard model is one of most successful theories.
But for example, fractional charge such as -1/3e and +2/3e have NOT been found, because they insist quarks and gluons can NEVER be isolated.
( And do you think the value of "-1/3" or "+2/3" is too good to be true ? Why they cannot be "2/5e" or "4/7e" ? )
Of course, if these unreal quarks don't exist, standard model is wrong.
So also in this case, only under very "limited" precondition that imaginary fractional charges exist, they are doing experiments about standard model.
If these preconditions change, of course, all these theories would collapse.
(Fig.6) Why the experiment of entanglement is always a single pattern ?

Unfortunately, experiments about entanglement have made no progress at all, I think.
They just repeat the same pattern of experiments such as "some loop hole is closed", or "four or three photons are entangled".
But as you feel, what we want to know most is the concrete mechanism how faster-than-light spooky link acts on each particle !
They try NOT to elucidate the true mechanism of entanglement itself, and just do "Shut up and calculate !".
Because they themselves notice entanglement itself is an illusion, and photons is just a classical waves, I think.
And as shown in this section, "not-coexisting" entangled photon is due to "photon particle".
(Fig.7) Present antimatter experiment is only a single pattern.
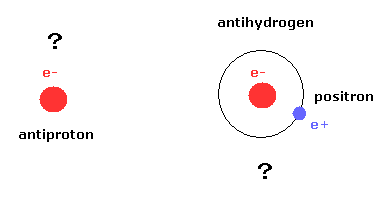
In conclusion, the present experiments of general relativity, entanglement, and particle physics are only repeating a single pattern, and making no progress, I think.
If you are so confident that general relativity is right, you need to investigate the true mechanism how "imaginary" gravitons can cause time dilation.
And you should elucidate the concrete relationship between virtual particles and these gravitons in the actual experiment.
If you are confident that antimatters including antiquarks really exist, at least, you need to generate antimatter from the vacuum itself, NOT using heavy metals !
Because using heavy metals means these phenomena are only electron, proton, or neutron captures.
( Actually, magnetic moment of "doubtful" positron is the same as that of electron. )
Generally, the amount of generated antimatters are very small, almost all of them are infinte noises.
They just estimate their existence only from final products of lights.
(Fig.8) True electron distribution of Neon (= Ne ).
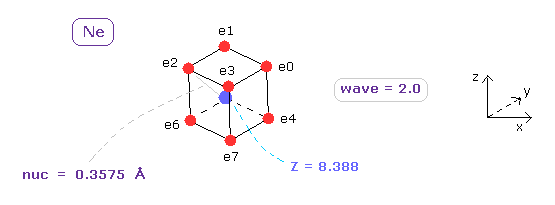
Here we compute the true atomic radius (= nuc ) and central charge Z using this program.
Save this program text as "twoion.java", and compile it, neglecting note, "--Xlint: unchecked ---".
Manipulating methods of this program is almost same as this page.
And computing method of true atomic radius is the same as this page.
In this program, based on total force (F) acting on each electron, we define "temporary" radius (r1), as follows,
(Eq.1)
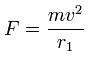
This means the centrifugal force is supposed to be equal to F.
"v" is the electron's velocity which value can be gotten from each kinetic energy.
And based on these values, we can find the values (wn) of de Broglie's waves, which is contained in one orbit of each electron, as follows,
(Eq.2)
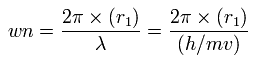
As shown in App. 1, total 1-8th ionization energy of neon is 953.614 eV.
Using Virial theorem, total potential energy (= tV ) becomes -953.614 × 2 = 1907.228 eV.
When total V is almost 1907.228 eV and de Broglie wave in one orbit (= Waves ) is 2.0, the distance (= nuc ) between each electron and Ne nucleus is 0.3575 Å and its central charge Z is +8.388.
( Here new unit of 1 MM = 1.0 × 10-14 meter is used. )
Choose "Ne" in the scroll bar, and click "Atom A" button.
"A-tV" at the lower right means total potential energy only inside A atom.
You can adjust this A-tV value to almost 1907.228 eV, chaging "nuc" and "charge Z".
( Enter some value into the designated textboxes, and click "nuc" or "charge Z" button. )
(Fig.9) True electron distribution of Fluorine (= F ).
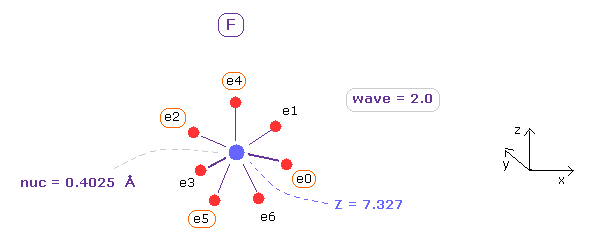
Next, choose "F" in the scroll bar, and click "Atom A" button.
As shown in App. 1, total 1-7th ionization energy of fluorine is 658.836 eV.
So accoding to Virial theorem, total potential energy (= A-tV ) needs to be almost 658.836 × 2 = 1317.672 eV.
When average de Broglie wave in one orbit (= ave ) is 2.0, distance between each electron and F nucleus (= nuc ) is 0.4025 Å (= 4025 MM ), and central charge Z = +7.327.
When you click "A nuc" button, force component acting on A nucleus (= F ) only inside A atom is displayed.
In this case, ( FX, FY, FZ ) = ( 3, 2, 3 ) are almost zero, and F nucleus is stable.
When you click "+X (MM)" button, you can change the coodinates of only electrons 0, 2, 4, and 5.
( electrons 1, 3 and 6 are automatically changed symmetrically based on ele 0, 2, 5. )
Adjusting "nuc", "charge Z" and each coodinate, you can find best symmetric (= lowest tV ) F electron distribution.
(Fig.10) PDB data bank - Potassium channel.
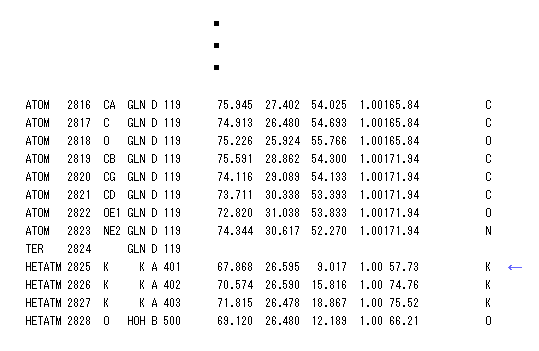
It is famous that potassium channel allows only potassium ion (= K+ ) through, and blocks smaller sodium ion (= Na+ ).
We investigate this reason using PDB data (= K-O distance ) and program above.
PDB (= protein data bank ) is very convenient and useful tool, combining with RasMol, which can visualize protein structure. ( So I want you to try it once. )
We search for "1BL8" (= K+ channel PDB id ) in PDB site, click Display File, save that PDB data, and display it using RasMol.
(Fig.11) K+ ion and oxygen atom (= electron pair donor ).
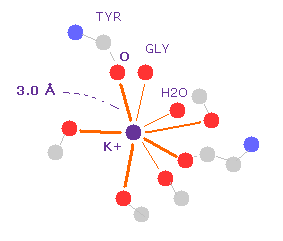
Number "401" residue of K+ channel is potassium ion ( see Fig.10 ).
So we display only atoms which are less than 5.0 Å far from K+ ion, using "select" command.
If we click K+ and one of oxygen atoms around it, we can know K-O distance is about 3.0 Å.
It is said only K+ ion can interact with 9 oxygen of potassium channel. On the other hand, smaller Na+ ion cannot.
But unrealistic quantum chemistry does NOT have concrete electron's position and the concept of "force" against another particle.
So we cannot know "what is actually happening" in detail using quantum chemistry.
(Fig.12) Positive force acting on O nucleus.
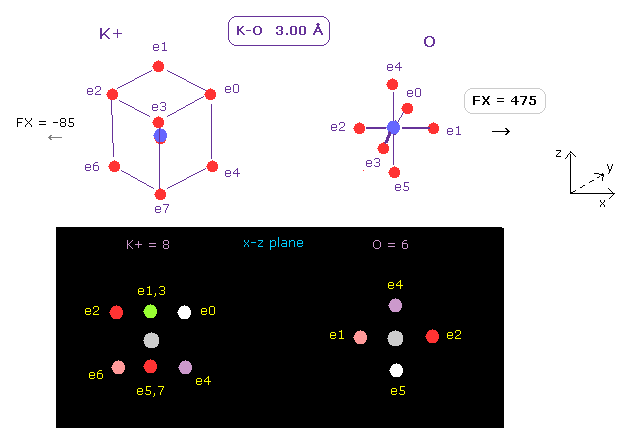
After running this program, we choose K+ (= potassium ion ) and O (= oxygen ) as A and B atoms, respectively.
And make internuclear distance 3.00 Å ( internuc = 30000 MM ).
Potassium ion (= K+ ) has eight valence electrons like Ar.
In the same way above, we can determine K+ electron orbital radius (= 0.5970 Å ) and its central charge (= 10.44 ) using 2-9th total ionization energy of App. 1.
As shown in Fig.12, repulsive force acting on each nucleus becomes FX = 475 and FX = -85, respectively.
(Fig.13) Repulsive force between O-H.
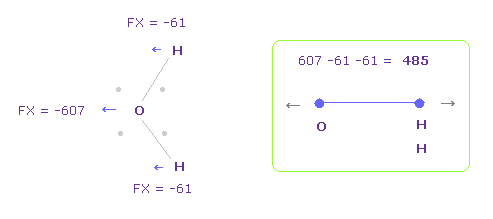
This repulsive force between K-O is almost same as H2O, as shown in this page (Fig.28).
| Molecule : | H2O | OF2 | OCl2 | OFH |
|---|---|---|---|---|
| Repulsive force: | 586 | 564 | 576 | 582 |
Furthermore, total repulsive force of K-O in Fig.12 is 475 + 85 = 560, which is almost same as other H2O-like molecule of this page (Table 6).
Positive repulsive force can attract negative electrons of oxygen ( for example, e4, e5 of Fig.12 ) enough, overcoming other atoms such as H.
(Fig.14) Force of Na+ ion is small.
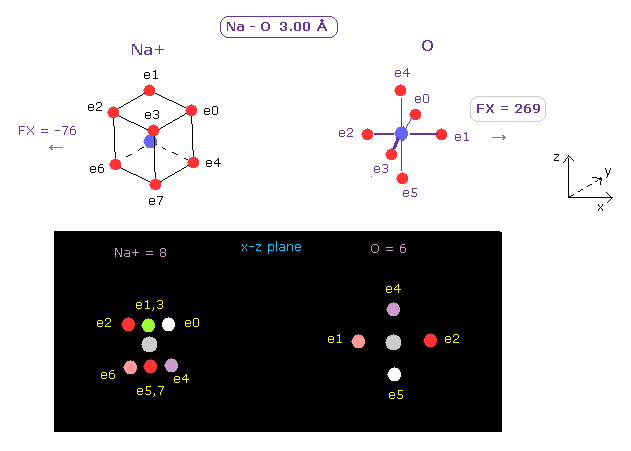
Next we choose Na+ as A atom. ( Choose "Na+" in the scroll bar, and click "Atom A" button. )
In this case , force acting on O nucleus is smaller ( FX = 269 ).
This means Na+ ion cannot attract oxygen electrons enough, which leads to the fact Na+ ion hydrate cannot release H2O to pass narrow K+ channel pore.
Basically Na+ and K+ ions exist as "hydrate" inside body, and need to release these attached H2O to pass narrow channel pore.
In K+ ion case, K+ can interact with pore oxygen enough to release H2O attached to it.
(Fig.15) [Na (H2O)]+ hydrate.
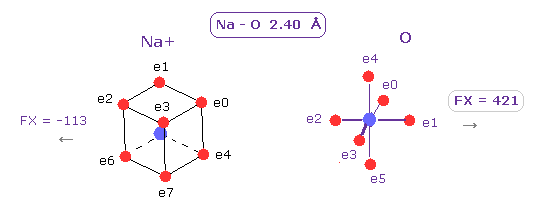
In usual [ Na+ - (H2O) ] hydrate, Na-O bond length is about 2.40 Å.
In this distance, force of Na+ ion against O nucleus becomes strong enough (= 421 ) to attract oxygen electrons.
It is said that K+ and Na+ ions have 1.33 Å and 0.95 Å radius.
But of course, quantum mechanics cannot show clear electron's position, so these radii do NOT mean true electron's distribution.
They are gotten from internuclear bond lenth.
Not having clear electron's distribution means quantum mechanics have no concept such as Coulomb force, though Coulomb force is obviously one of crucial factors in various bonds.
(Fig.16) Force = 1000.
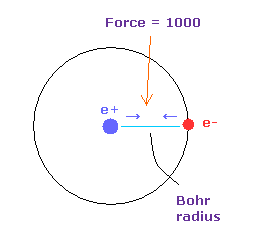
Force unit used in this page is shown in Fig.16.
The force between electron and nucleus in H atom ground state is supposed to be "1000".
So when the distance between electron and +e nucleus is Bohr radius, its force becomes 1000.
For example the force of 1468 is 1.468 times that in H atom ground state.
(Fig.17) K-O is not a covalent bond.
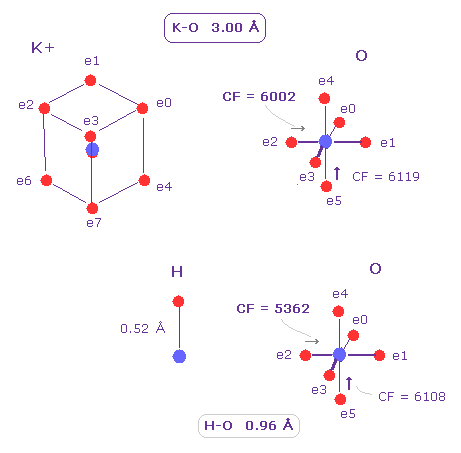
The big positive nucleus of K+ ion is exposed, because its valence electrons are widely distributed (= 3.0 de Broglie wavelength ).
So even if K-O length is as long as 3.00Å, it can interact with O nucleus.
But different from usual covalent bond of O-H, direct force acting on valence electron (= e2 ) of oxygen is smaller.
CF = 6002 ( = force toward O nucleus ) is bigger than that of O-H covalent bond (= 5362 ).
This means the influence of K+ is smaller than O-H bond case.
If K+ ion binds tightly to oxygen of potassium channel pore, its pore is plugged. This is a serious problem.
So K-O length of 3.0 Å is proper value for its biological system.
(Fig.18) PDB data bank - Myoglobin.
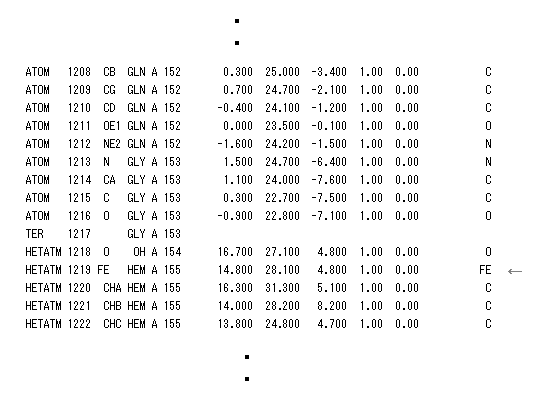
It is known that myoglobin is oxygen-binding protein having porphyrin ring with an iron ( Fe2+ ) center like hemoglobin.
So this structure of myoglobin with Fe2+ needs to be stable, even when it is attacked by strong oxygen.
We search for "1MBN" (= myoglobin PDB ID ) in PDB site, click Display File, save that PDB data, and display it using RasMol.
Number 155 residue is Fe atom, so we display only atoms which are less than 4.0 Å far from Fe2+ ion, using "select" command.
Other values means the ( x, y, z ) coordinate (= Å ) of each atom.
(Fig.19) Fe2+ ion and N, O atoms.
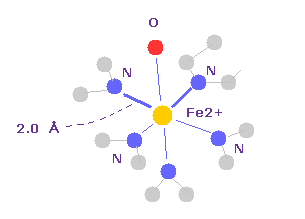
Fe2+ has six valence electrons, and forms octahedral structure with other 5 × N + O atoms.
Octahedral structure is very common form in matallic coodination compounds.
It means classical Coulomb force is always important instead of imaginary s,p,d orbitals.
If we click Fe and one of nitrogen atoms around it, we can know N-O distance is about 2.0 Å.
(Fig.20) Force acting on e3 of nitrogen
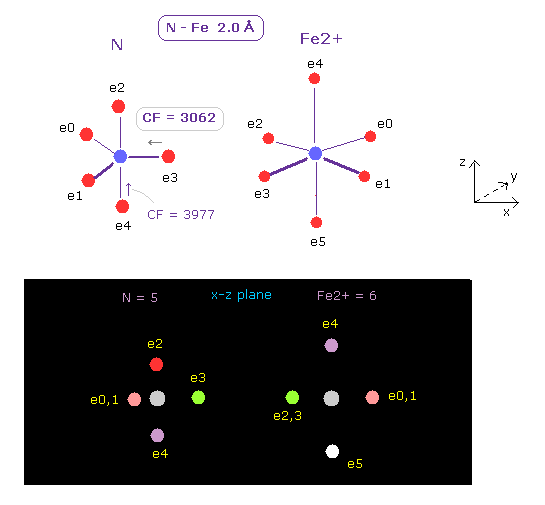
After running this program, we choose N (= nitrogen ) and Fe2+ (= iron ion ) as A and B atoms, respectively.
And make internuclear distance is 2.00 Å ( internuc = 20000 MM ).
Iron ion (= Fe2+ ) has six valence electrons.
In the same way above, we can determine Fe2+ electron orbital radius (= 0.8264 Å ) and its central charge (= 11.91 ) using 3-8th total ionization energy of App. 1.
As shown in Fig.20, positive force of Fe2+ is very strong, so CF (= force against e3 toward N nucleus ) is decreased to 3062.
(Fig.21) Comparison of Force acting on e3 of nitrogen.
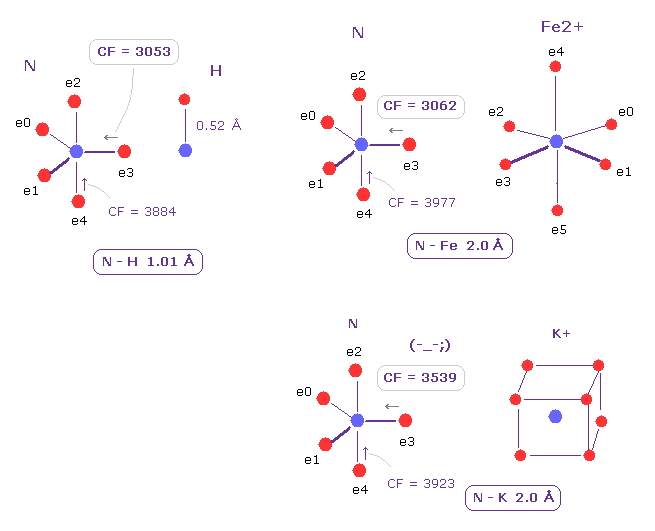
Choosing ( N,H ), ( N, Fe2+ ), and ( N, K+ ) as A,B atoms, we compare the influences on valence electron e3 of nitrogen.
As shown in Fig.21, N-Fe2+ bond is almost same as usual N-H bond with respect to CF acting on e3 ( CF = 3062, 3053, respectively ).
This means Fe2+ forms "tight" covalent bonds with nitrogen, and its bond is very stable.
On the other hand, if you change Fe2+ into K+ ion, its influence on e3 electron becomes weaker.
So Fe2+ has very great influence on protein configuration due to its big positive nucleus.
(Fig.22) Comparison of force (= CF ) acting on "e1" electron of oxygen.
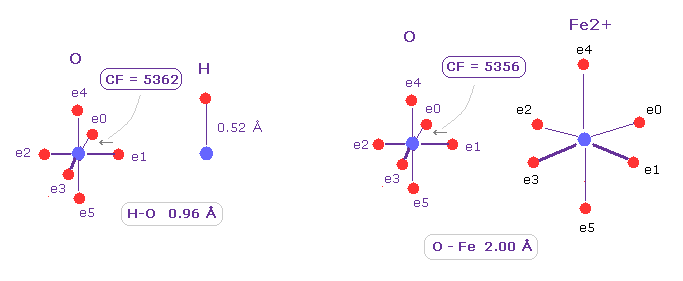
Also in bond with oxygen, Fe-O is covalent-like.
Choose ( O,H ) and ( O Fe2+ ) as A,B atoms, rotate O atom by 45 degree on x-y plane.
As shown in Fig.22, forces acting on e1 valence electron of oxygen are almost same in O-H and O-Fe bonds ( 5362, 5356, respectively ).
In conclusion, Fe2+ ion forms tight covalent bonds with nitrogen and oxygen atoms in myoglobin or hemoglobin, and is stable even when it is attacked by other oxygen.
(Fig.23) Comparison of forces (= CF ) acting on "e1" electron of oxygen.
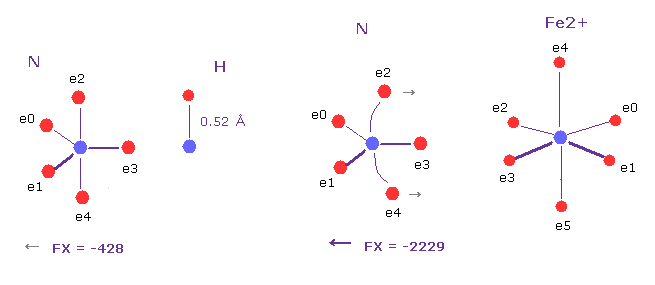
Basically, big positive metallic ions have great influences on overall protein conformation.
In the above cases, Fe2+ exerts great positive electric power against N nucleus ( compare FX =-2229 with -428 of N-H bond ).
As a result, electron e2 and e4 are attracted a little toward Fe2+, which influences other atoms. ( Try moving them. )
Quantum mechanics insists N and O act like electron donor, because they have non-binding electron pair.
But as you see Fig.23, electrons e2 and e3 are clearly at opposite positions of N nucleus.
So very abstract concept of "electron pair" is misguided.
Basically, iron ion is Fe2+ inside body.
Fe3+ is almost inactive foming tight bonds with other atoms.
( So Fe2O3 is very stable and insoluble. )
Force CF acting on valence electrons of Fe2+ is almost "4000", so there is a little room for removing another electron.
(Fig.24) Calmodulin- Ca2+ complex
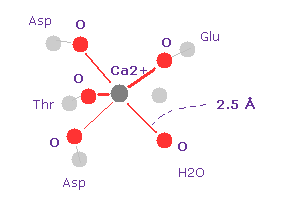
Calmodulin is a calcium-binding messenger protein, that transduces calcium signals by binding calcium ions and confomational change.
Fig.24 shows Ca2+ ion binding structure in calmodulin ( PDB id = 1CLL ).
The distance between Ca2+ and oxygen atom is about 2.5 Å.
After running this program, we choose O and Ca2+ as A,B atoms.
Making internuclear distane 2.5 Å (= 25000 MM ), and rotate oxygen by 45 degree on x-y plane.
(Fig.25) Ca2+ has "intermediate" influence.
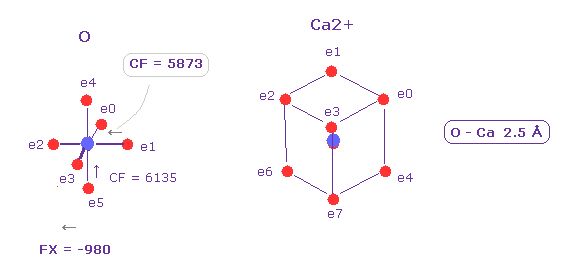
Ca2+ has eight valence electrons and "3" de Broglie wavelegnth.
In the same way, we compute radius (= 0.5277 Å ) and central charge (= 11.494 ) based on total ionization energy.
As shown in Fig.25, Ca2+ ion has intermedeate influences between K+ and Fe2+ ions.
Positive force acting on another O nucleus is FX = 980, which is between 475 (= K+, Fig.12) and 2000 (= Fe2+, Fig.23).
And CF on e1 valence electron becomes 5873, which is between 6002 (= K+, Fig.17 ) and 5356 (= Fe2+, Fig.22 ).
So calcium ion has no power enough to form covalent bond ( considering also closed 8 electrons ), but can change protein conformation as rapid signal transmission.
If they stick to oxygen forming covalent bonds, they cannot transmit signal.
(Fig.26) Mg2+ ion in chlorophill.
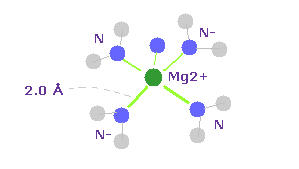
Magnesium ion (= Mg2+ ) is used in chlorophill and abosorbs light in photosynthesis.
As shown in Fig.26, the distance between Mg2+ ion and surrounding N atoms is about 2.0 Å ( PDB id= 2BHW ).
Mg has two valence electrons which are easily removed by two surrounding N (= nitrogen ).
As a result, Mg2+ has eight valence electrons like neon.
We use these ionization energies to compute orbital radius
(Fig.27) Influence by Mg2+ ion.
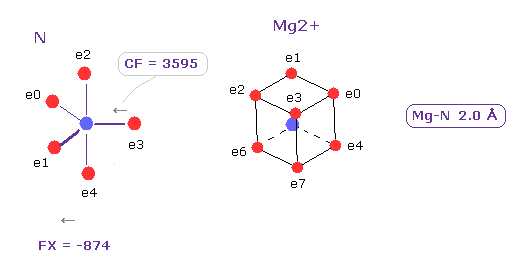
After running this program, we choose N and Mg2+ as A,B atoms, and make internuclear distance 2.0 Å (= 20000 MM ).
Influence of Mg2+ is weaker than usual covalent bond
Compare CF = 3595 with Fe2+ (= 3062, Fig.21 ).
Actually Mg-N bond is unstable, and Mg is easily replaced by other atoms under some condition.
(Fig.28) Why Magnesium ?
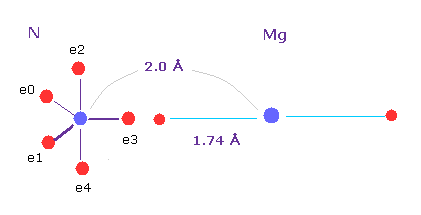
If we choose neutral "Mg", the distance between Mg nucleus and its valence electrons is 1.74 Å, which is almost same as Mg-N distance (= 2.0 Å ).
So two valence electrons of magnesium are easily released from Mg, and these electrons are used energy transmission in photosynthesis.
As a result, considering intermediate force of Mg2+, two loosly bound electrons, and light absorption, Mg was chosen in chlorophill, I think.
(Fig.29) Zn2+ ion in zinc-finger.
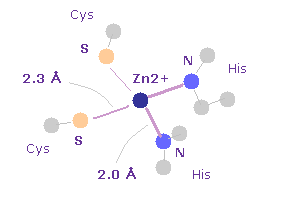
Basically small proteins are unstable, because they cannot wrap hydrophobic proteins enough using other proteins.
Though zinc-finger protein is very small, they are very stable, because very strong force of zinc ion keeps their structure.
As shown in Fig.29, the distance between Zn2+ ion and surrounding N atoms is about 2.0 Å ( PDB id= 1ZAA ).
Zn has 12 valence electrons, so Zn2+ has 10 valence electrons.
We use these ionization energies to compute orbital radius
(Fig.30) Influence by Zn2+ ion.
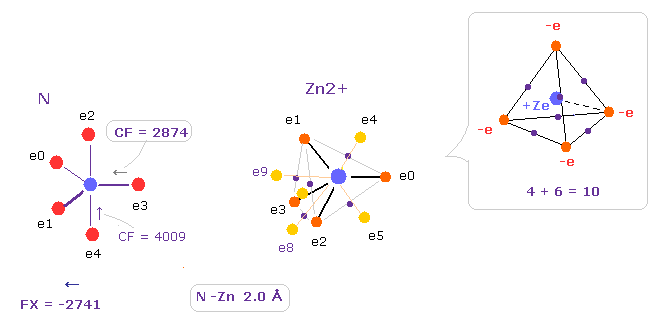
After running this program, we choose N and Zn2+ as A,B atoms, and make internuclear distance 2.0 Å (= 20000 MM ).
10 valence electrons are arranged by combining 4 vertices and 6 middle points of each side in tetrahedron.
Influence of Zn+ is stronger than Fe2+ ion.
Compare CF = 2874 with Fe2+ (= 3062, Fig.21 ).
This means e3 electron of nitrogen is more attracted toward Zn2+ ion than Fe2+
(Fig.31) Force of Zn2+ ion against sulfur.
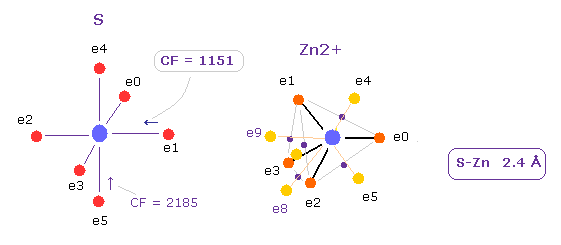
The distance between S and Zn2+ ion is 2.4 Å.
In this case, force action of e1 electron toward S nucleus becomes CF = 1151.
This value shows attractive force of Zn2+ ion is very strong.
(Fig.32) N=C, S=C double bonds.
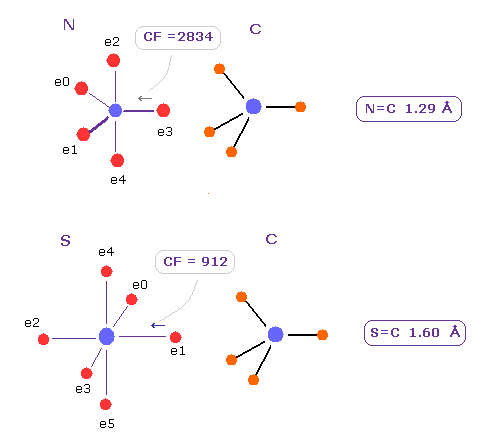
Influences of Zn2+ ion upon nitrogen and sulfur are very similar to double bonds of N=C and S=C.
CF of N=C is 2834, which is close to 2874 in N-Zn bond of Fig.30.
CF of S=C is 912, which is close to 1151 in S-Zn bond of Fig.31.
Difference between them are Zn2+ ion has big positive nucleus, so its influence spreads over wide area, which causes overall protein's structual change.
So strong attractive force of zinc ion is a important key player to keep small zinc protein's structure stable even in various strict conditions.
(Fig.33) Cu ion in hemocyanin.
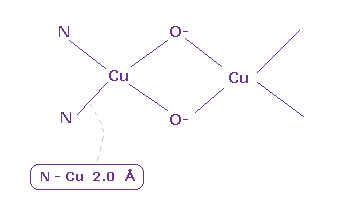
Hemocyanin are proteins that transport oxygen throughout the bodies of some invertebrate animals.
Basically a copper exists as Cu+ ion and becomes Cu2+ ion, when oxygen binds to it.
According to protein structure ( PBD id = 1LNL ), average Cu-N (= nitrogen ) bond length is 2.0 Å.
(Fig.34) Influence of Cu+ ion upon nitrogen .
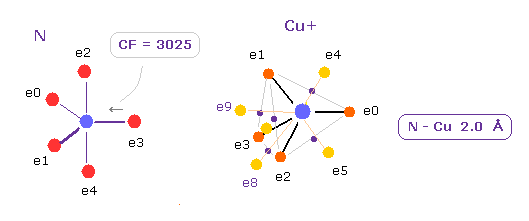
Cu has 11 valence electrons, so Cu+ ion has 10 valence electrons.
Probably the number of "10" is related to a symmetric ( see Fig.30 ), and stable structure.
As shown in Fig.34, the force CF acting on e3 electron is decreased to CF= 3025 due to Cu+ attractive force.
This value of 3025 is very cloce to CF= 3062 in Fe2+ case ( see Fig.21 ).
So Cu+ has almost same property as Fe2+ ion.
If you change Cu+ into Cu2+, this CF is slightly decreased to 2950.
(Fig.35) Various metallic ions
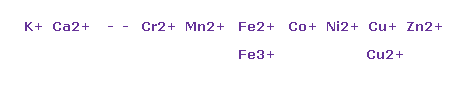
In metals, "+2" ions are most popular form.
Basically, in "4" group, forces attracting valence electrons by central charge become weaker, because their orbittal radius is long (= "4" de Broglie wavelength ).
So each atoms tend to release as much as two valence electrons due to these weak binding force.
But removing 3 or 4 valence electrons are difficult due to their larger positive charge.
Copper tends to be Cu+ ion, and Zn tends to be Zn2+ ion.
These are related to "symmetric" structure of "10" electrons, as shown in Fig.30.
Other metals such as manganase (= Mn ), nickel (= Ni ) and selenium (= Se ) are known to be used in various enzymes such as SOD and urease.
Try investigating these functions using above program, too.
When there are more than 8 valence electrons, textboxes of more than ele 7 electros are displayed on the screen, if you click "ele No" button.
If you click "+X (MM)" button, in some atoms, electrons you can manipulate are restricted.
You can manipulate only electrons to which word of "ele" is attached.
( Other electrons' coordinates are automatically determined based on manipulated electrons. )
When you click "A nuc" button, force acting on each nucleus displayed is changed.
Basically, force components (= FX, FY, FX ) shown on textbox are overall force acting on each A or B nucleus considering All influences of A anb B nuclei and electrons.
( For example, "A only" means force computed considering only A atom's electrons and nucleus. ).
"x-y and", "x-z ang", and "y-z ang" buttons rotate each atom by the designated angle (= degree ) on x-y, x-z, or y-z planes.
"A-tV" and "B-tV" mean total potential energy only inside A atom ( or only B atom ).
If you click "AB change" button, atom displayed on textboxes is changed between A and B atoms.
Other methods are same as this page.
| Atom | 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | 10th | 11th | 12th | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | 13.606 | - | - | - | - | - | - | - | - | - | - | - | 13.606 |
| Be | 9.323 | 18.211 | - | - | - | - | - | - | - | - | - | - | 27.534 |
| B | 8.298 | 25.155 | 37.931 | - | - | - | - | - | - | - | - | - | 71.384 |
| C | 11.260 | 24.383 | 47.888 | 64.494 | - | - | - | - | - | - | - | - | 148.025 |
| O | 13.618 | 35.121 | 54.936 | 77.414 | 113.899 | 138.120 | - | - | - | - | - | - | 433.108 |
| F | 17.423 | 34.971 | 62.708 | 87.140 | 114.243 | 157.165 | 185.186 | - | - | - | - | - | 658.836 |
| Ne | 21.565 | 40.963 | 63.45 | 97.12 | 126.21 | 157.93 | 207.276 | 239.100 | - | - | - | - | 953.614 |
| Na | 5.139 | - | - | - | - | - | - | - | - | - | - | - | 5.139 |
| Na+ | - | 47.286 | 71.62 | 98.91 | 138.4 | 172.18 | 208.50 | 264.25 | 299.864 | - | - | - | 1301.01 |
| Mg | 7.646 | 15.035 | - | - | - | - | - | - | - | - | - | - | 22.681 |
| Mg2+ | - | - | 80.144 | 109.266 | 141.27 | 186.76 | 225.02 | 265.96 | 328.06 | 367.50 | - | - | 1703.98 |
| Al | 5.986 | 18.829 | 28.448 | - | - | - | - | - | - | - | - | - | 53.263 |
| Al3+ | - | - | - | 119.992 | 153.825 | 190.49 | 241.76 | 284.66 | 330.13 | 398.75 | 442.00 | - | 2161.607 |
| Si | 8.152 | 16.346 | 33.49 | 45.142 | - | - | - | - | - | - | - | - | 103.13 |
| P | 10.487 | 19.77 | 30.202 | 51.444 | 65.025 | - | - | - | - | - | - | - | 176.928 |
| S | 10.36 | 23.338 | 34.79 | 47.222 | 72.595 | 88.053 | - | - | - | - | - | - | 276.358 |
| Cl | 12.968 | 23.814 | 39.61 | 53.465 | 67.8 | 97.03 | 114.196 | - | - | - | - | - | 408.883 |
| Atom | 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | 10th | 11th | 12th | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K | 4.341 | - | - | - | - | - | - | - | - | - | - | - | 4.341 |
| K+ | - | 31.63 | 45.806 | 60.91 | 82.66 | 99.4 | 117.56 | 154.88 | 175.817 | - | - | - | 768.663 |
| Ca | 6.113 | 11.872 | - | - | - | - | - | - | - | - | - | - | 17.985 |
| Ca2+ | - | - | 50.913 | 67.27 | 84.50 | 108.78 | 127.2 | 147.24 | 188.54 | 211.275 | - | - | 985.718 |
| Ti | 6.828 | 13.576 | 27.492 | 43.267 | - | - | - | - | - | - | - | - | 91.163 |
| Cr | 6.767 | 16.486 | 30.96 | 49.16 | 69.46 | 90.635 | - | - | - | - | - | - | 263.468 |
| Cr2+ | - | - | 30.96 | 49.16 | 69.46 | 90.635 | - | - | - | - | - | - | 240.125 |
| Mn | 7.434 | 15.64 | 33.668 | 51.2 | 72.4 | 95.6 | 119.203 | - | - | - | - | - | 395.145 |
| Mn2+ | - | - | 33.668 | 51.2 | 72.4 | 95.6 | 119.203 | - | - | - | - | - | 372.071 |
| Fe | 7.902 | 16.188 | 30.652 | 54.8 | 75.0 | 99.1 | 124.98 | 151.06 | - | - | - | - | 559.682 |
| Fe2+ | - | - | 30.652 | 54.8 | 75.0 | 99.1 | 124.98 | 151.06 | - | - | - | - | 535.592 |
| Fe3+ | - | - | - | 54.8 | 75.0 | 99.1 | 124.98 | 151.06 | - | - | - | - | 504.94 |
| Co | 7.881 | 17.084 | 33.50 | 51.3 | 79.5 | 102.0 | 128.9 | 157.8 | 186.13 | - | - | - | 764.095 |
| Co+ | - | 17.084 | 33.50 | 51.3 | 79.5 | 102.0 | 128.9 | 157.8 | 186.13 | - | - | - | 756.214 |
| Ni | 7.640 | 18.169 | 35.19 | 54.9 | 76.06 | 108 | 133 | 162 | 193 | 224.6 | - | - | 1012.559 |
| Ni2+ | - | - | 35.19 | 54.9 | 76.06 | 108 | 133 | 162 | 193 | 224.6 | - | - | 986.75 |
| Cu | 7.726 | 20.292 | 36.841 | 57.38 | 79.8 | 103 | 139 | 166 | 199 | 232 | 265.3 | - | 1306.339 |
| Cu+ | - | 20.292 | 36.841 | 57.38 | 79.8 | 103 | 139 | 166 | 199 | 232 | 265.3 | - | 1298.613 |
| Cu2+ | - | - | 36.841 | 57.38 | 79.8 | 103 | 139 | 166 | 199 | 232 | 265.3 | - | 1278.321 |
| Zn | 9.394 | 17.964 | 39.723 | 59.4 | 82.6 | 108 | 134 | 174 | 203 | 238 | 274 | 310.8 | 1650.88 |
| Zn2+ | - | - | 39.723 | 59.4 | 82.6 | 108 | 134 | 174 | 203 | 238 | 274 | 310.8 | 1623.522 |

2013/6/1 updated. Feel free to link to this site.