 (2/9/2026) Quantum internet, quantum computer fraud
(2/9/2026) Quantum internet, quantum computer fraudLink
 (1/6/2026) Einstein relativity is wrong
(1/6/2026) Einstein relativity is wrong
Youhei Tsubono, Japan


Contents (12/14/2025) 
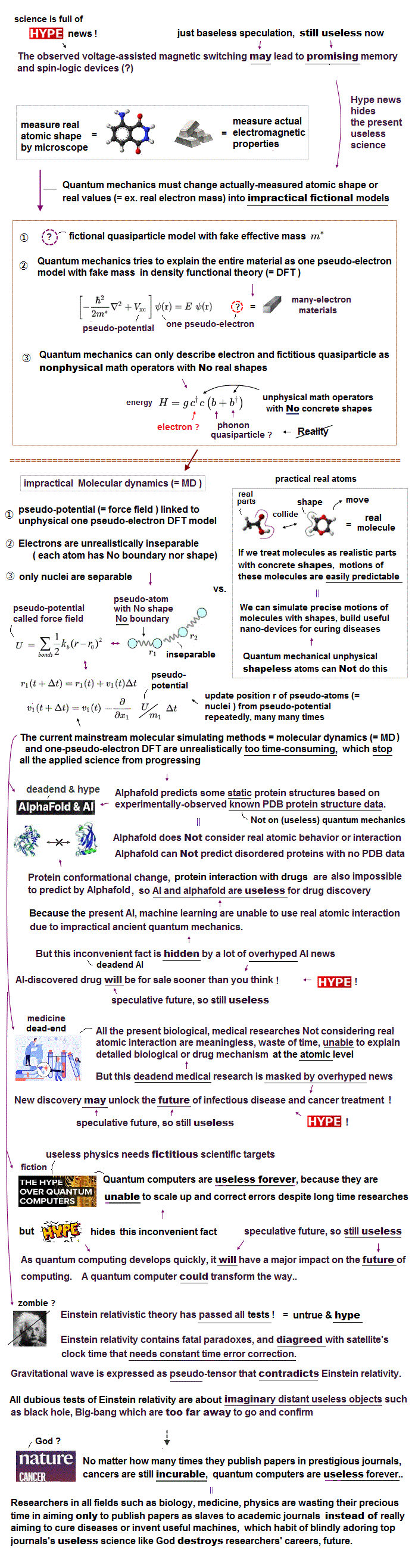
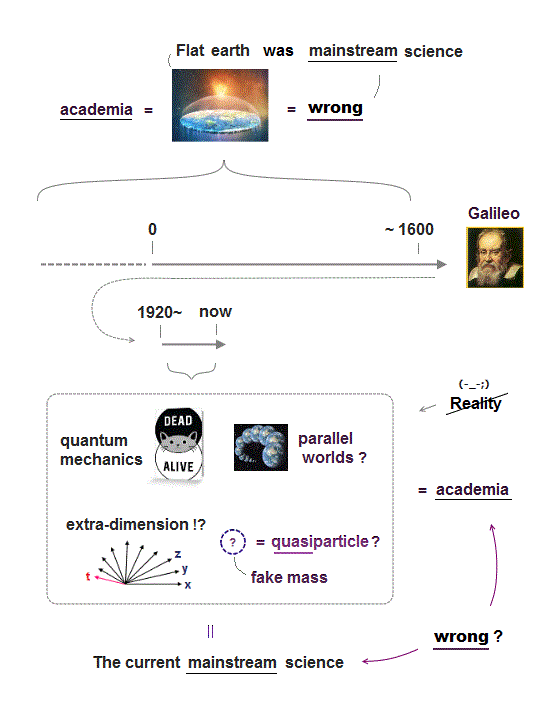
(Fig.1) Quantum mechanics is wrong, hampering curing cancers, Alzhemier. Overhyped AI, quantum computers are useless forever
(Fig.1') Today's wrong mainstream science just makes our life unhappy, inconvenient. ↓
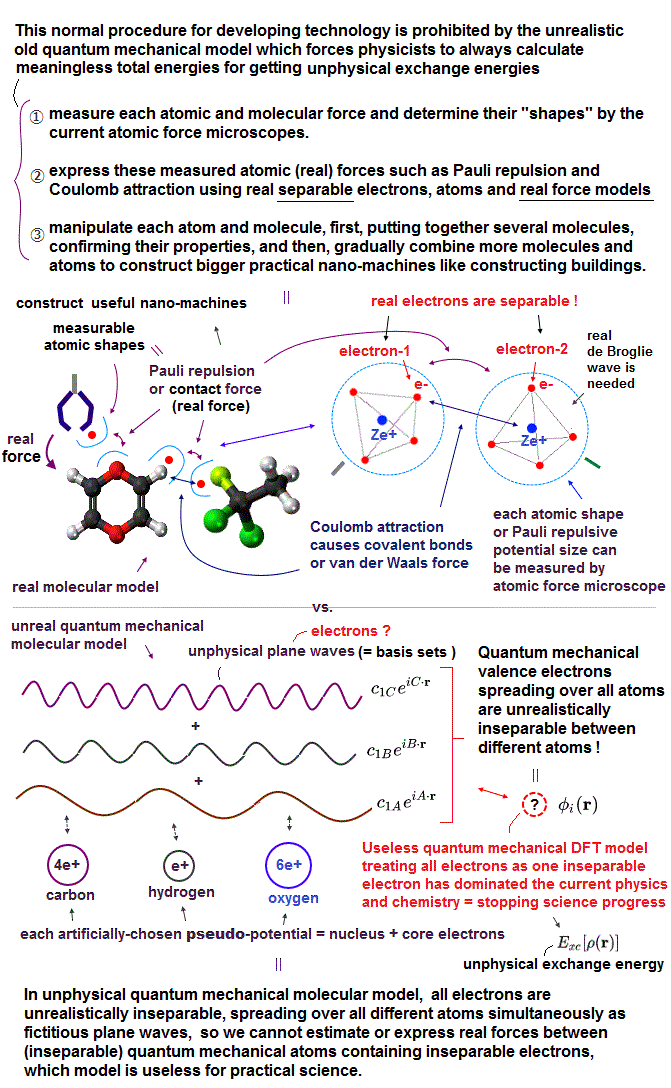
(Fig.1a) Quantum mechanical unreal atomic model prevents curing diseases.
Many serious diseases such as Alzheimer, dementia, cancers, HIV, paralysis, autoimmune diseases.. are still incurable despite extremely long years and enormous money spent for their medical researches.
Because today's medical researches are deadend, unable to utilize or clarify the atomic mechanisms of biological reactions for curing diseases, due to today's useless atomic theory called quantum mechanics that hampers microscope technology and AI.
Only overhyped fake news is rampant.
Due to useless Schrödinger equation that cannot predict any multi-electron atoms, quantum mechanics has to treat multi-electron molecules and material as one pseudo-electron density functional theory (= DFT ) or fictional quasiparticle model with fake negative mass and fractional charge lacking real atomic shape that hampers nano-technology.
Contrary to an incredible amount of overhyped news, quantum mechanics has been completely useless for any technologies such as transistors, medicine, laser, quantum computers based on unreal parallel-universe simultaneous calculations ( this-Challenges and the road ahead, this-lower-Challenges ), modern MRI, quantum sensors, spintronics, just making our life unhappy.
The 3rd-paragraph of this (2025) says -- Useless quantum mechanics
"Our analysis shows No consistent evidence that quantum algorithms outperform classical methods for clinical decision-making or health service delivery" ← Quantum mechanics has been useless for medicine and health care contrary to overhyped misleading news.
Today's medical researches use only macroscopic biological tools (= Not looking into atomic mechanisms ) such as PCR, restriction enzymes obtained from bacteria, immunofluorescence by animals' antibodies, DNA, RNA, CRISPR of bacterial immune system, GFP of jellyfish, all of which were obtained from natural organisms, Not designed by humans nor (useless) quantum mechanics.
No quantum mechanical Schrödinger equation nor DFT methods are used in today's medical or biological researches ( this-p.2-Materials and methods, this-p.8-9-Material and methods, this-p.8-9-Methods, this-p.2-p.4 ) in contrast to the (impractical) physics researches ( this-p.8-left-Schrodinger-equation, this-p.7-methods-DFT, this-p.5-right-DFT calculations ).
(Fig.M) Quantum mechanics with fictional parallel universes is a useless pseudo-science.
Quantum mechanical Schrödinger equations are useless, cannot predict energies of any multi-electron atoms nor molecules.
Quantum mechanics has to treat any multi-electron molecules or proteins as fake quasiparticle or one-pseudo-electron DFT model with unreal (effective) mass and charge lacking real atomic shape, which is completely useless for medicine.
Quantum mechanical unreal useless concepts such as parallel-world superposition, superluminal entanglement (= sending No real information ), tunneling by negative kinetic energy are irrelevant to actual medicine or biology.
This-p.8-conclusions say (in 2023 ) -- Useless quantum mechanics
"Although
quantum theories require more technological advancements and further research before
their practical application can be achieved" ← quantum mechanics still has No practical application in medicine ( this-2nd-last-paragraph ).
Even this news overhyping ( hopeless ) quantum computers and quantum
dots admits in its lower Bottom line (in 2025 ),
"While many applications remain in early research phases," ← still quantum mechanics is useless in medicine.
Even this overhyped news-lower-Nanotechnology and quantum dots in cancer treatment (in 2025) says
"The use of quantum dots in cancer treatment is still in its early stages" ← still useless
This or this (in 2025 ) says -- No cure Alzheimer
"The exact cause remains unclear,"
"Currently, there is No cure for Alzheimer's disease," ← Quantum mechanics is proven completely useless for clarifying Alzheimer disease mechanism after 100 years of fruitless researches ( this-p.2-left-2. ).
This-introduction-2nd-paragraph (in 2025 ) says -- Incurable cancer
"there are still numerous challenges that hinder successful treatment outcomes. These challenges primarily arise from an incomplete understanding of the precise mechanisms underlying cancer pathogenesis"
↑ The impractical quantum mechanics is also proven helpless for clarifying and curing cancers.
The overhyped quantum biology allegedly using ( fictional ) quantum superposition parallel worlds and quasiparticles with unreal mass and charge ( this-3rd-paragraph ) is also useless, completely irrelevant to actual biology or medicine.
This-5th-paragraph says -- Useless quantum biology
"Another reason quantum biology is Not considered a legitimate field of science "
This-Quantum biology today says -- Useless quantum mechanics
"physicists of the 20th century believed that quantum effects had No significant impact on biology."
This-middle-The Skeptics:.. (2025) says -- No quantum mechanics
"Several researchers in biophysics and molecular biology question whether quantum effects truly play a functional role in biological systems"
This recent researgh-p.9-left-last-paragraph (in 2024) say
"the idea of
quantum processes in the brain remains largely speculative and
controversial."
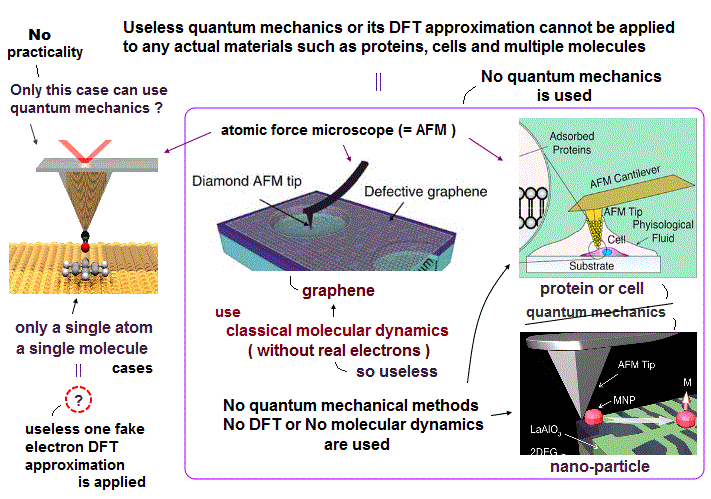
(Fig.E) Only multi-probe atomic force microscopes can get subatomic resolution (= see smaller than a single atom ) and clarify atomic mechanisms of diseases, which is hampered by unreal quantum mechanical atomic model.
Today's medical researches, which use only macroscopic biological tools unable to clarify atomic mechanisms of diseases, have been deadend with No power to cure cancers, Alzheimer, HIV..
The best mainstream microscopes used in medical researches have been (cryogenic) electron microscopes since 1933 ( cryo-electron microscope or cryo-EM has been used since 1970s as the current mainstream best microscope, this-brief history ).
X-ray crystallography (= imaging proteins' structures by seeing X-ray diffraction or interference scattered by the target orderly-crystallized proteins ) is useless, cannot see proteins that cannot be crystallized.
NMR (= vaguely measuring nuclear magnetic field of atoms irrelevant to quantum mechanics ) can see only very small molecules ( this-middle-Disadvantages of NMR, this-4th-paragraph, this-lower-table-disadvantage ).
This-2nd-last-paragraph (2025) says -- Useless experiments
"Membrane proteins can't easily be imaged by x-ray crystallography, the other workhorse protein imaging technique, because they don't readily assemble into the crystals that x-ray imaging requires. The scarcity of membrane protein structures also hampers artificial intelligence tools for structure prediction, which have to be trained on known structures "
Cryogenic electron microscopy is the current best mainstream method for (vaguely) imaging and averaging the target proteins' structures by measuring electrons randomly collided with proteins (= even this current best microscope cannot see the native or flexibly-changing structures of proteins, so useless, this-p.4-p.5, this-2nd~4th-paragraphs ).
↑ This current best cryo-electron microscope (= cryo-EM ) is also useless ( this-Limitations ), can Not clarify atomic structures of proteins or diseases, because the resolution of the cryo-electron microscope is bad (> 3Å = 3 angstrom, this-middle-figure, this-3rd-paragraph-5.8Å resolution, this-p.5~5Å resolution ), which cannot see a single atom (< 1Å = 1 angstrom = 0.1nm ).
This-p.11-conclusion says -- Bad resolution
"However, it cannot be
ignored that cryo-EM still has certain limitations in drug
research"
"Although the current highest resolution
reached 1.2 Å. most of the reported cryo-EM structures
are still at the 3–4 Å level (= cannot get atomic < 1Å resolution )"
This-lower-Challenges and limitations say -- Useless cryo-EM
"The inherent intra-particle flexibility also remains a limiting factor in cryo-EM, which can lead to blurring and difficulty in achieving high-resolution structures."
↑ So the cryo-electron microscope with bad resolution is the main culprit keeping the current medical research deadend, unable to cure diseases.
AI, Alphafold trained on these current incorrect experimental protein structures are also useless, cannot predict exact proteins' behavior.
Only atomic force microscope (= AFM ), which was invented in 1990s, can see subatomic structures (= smaller than an atom ) with better resolution (< 1Å ) and manipulate single atoms ( this-p.2-p.7 ).
This-p.8-right-2. says -- AFM seeing atoms
"to our knowledge, AFM (= atomic force microscope ) is the only tool that allows to
see structure within an atom... show subatomically resolved
images of a single atom Si tip and a W tip, the bottom
row demonstrates subatomic resolution of a Fe and a Cu
adatom on Cu(111) observed with a CO terminated tip"
This-p.3-right-atomic resolution imaging says -- Atomic resolution
"One of the most widely used applications of AFM (= atomic force microscope ) is its
ability to image material surfaces at the sub-Angstrom (= seeing smaller than an atom < 1Å, this-p.64 ) level."
Atomic force microscopes used in the current medical researches have only dull tips that cannot see atoms.
Because today's atomic force microscope with only one useless probe tip cannot see 3-dimensional atomic structure ( this-lower-challenges ), because one probe alone cannot fix the target molecules stably.
Only multi-probe atomic force microscopes can see and clarify the detailed 3-dimensional atomic structures of proteins and cure diseases.
But the current mainstream unreal quantum mechanical atomic model such as useless Schrodinger equations and density functional theory (= DFT = impractical, this-p.8-p.9 ) can only treat molecules or proteins as fictitious quasiparticle or one pseudo-electron model lacking real atomic shapes, which hampers developing useful multi-probe atomic force microscopes that need much simpler real atomic models treating molecules as real objects with shapes.
↑ Useful multi-probe atomic force microscopes treating target molecules as real objects with shape will make quantum mechanical unreal shapeless model unnecessary. ← To protect the old quantum mechanical model, they intentionally try Not to develop useful multi-probe atomic force microscopes though they already have that technology.
So we should immediately stop using the impractical quantum mechanical shapeless atomic model and develop useful multi-probe atomic force microscopes (= able to manipulate and break target proteins gradually to see inner atomic structures of proteins, this or this-p.17-p.24 ) to clarify atomic mechanisms and cure diseases.
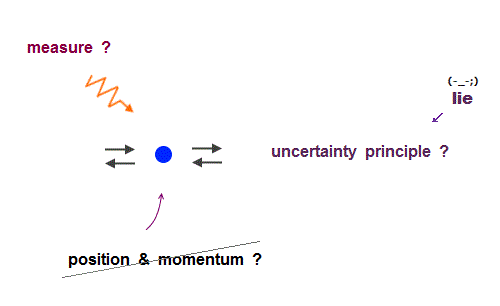
(Fig.2) Quantum mechanical hydrogen atom is unreal due to superposition, parallel universes, zero angular momentum, negative kinetic energy, which should be replaced by the realistic Bohr model.
Today's flawed useless quantum mechanics atomic theory is based on wrong probability atomic wavefunctions where each electron must unrealistically exist in all different places at once by using fictional superposition, unreal parallel universes and negative kinetic energy.
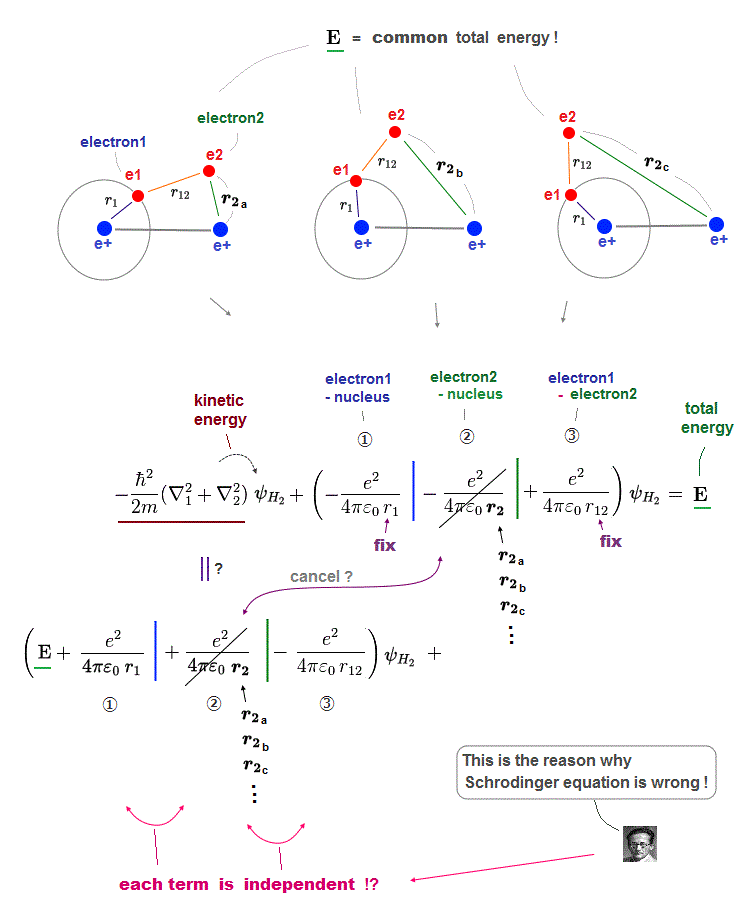
In this quantum mechanical Schrödinger equations, the atomic total energy E is equal to the sum of electrons' kinetic energy (= expressed as derivative of wavefunctions ) and Coulomb potential energy among all electrons and nuclei.
↑ Solving this Schrodinger equation (= finding true atomic wavefunctions conserving the constant total energy E in any electrons' positions ) is the only way for quantum mechanics to predict atomic energy, which is impossible in multi-electron atoms.
If each single electron exists in only one place, its probability wavefunction (= whose wave amplitude expresses the probability of an electron existing in that place ) increases in only that place, which increases the electron's kinetic energy (= differentiation of wavefunction ) unrealistically due to the sharper probability wavefunction (= shorter de Broglie wavelength ).
↑ So quantum mechanics unrealistically claims each electron must always exist in all places in all space simultaneously by (fictional) quantum superposition or illusory parallel universes (= expressed as spreading probability wavefunction ), which disproves quantum mechanics.
↑ The only way to eliminate these unreal quantum mechanical parallel universes is to reject the wave-particle duality or probability wavefunctions, and separate a real particle (= ex. electron ) and de Broglie wave in the medium.
Quantum mechanics or its unphysical atomic wavefunctions still lack reality, as this 4th-paragraph (7/30/2025) says
"However, no one knows what is happening in the physical reality behind the mathematics (= quantum mechanics can Not clarify real atomic mechanism, so useless, cannot cure diseases )."
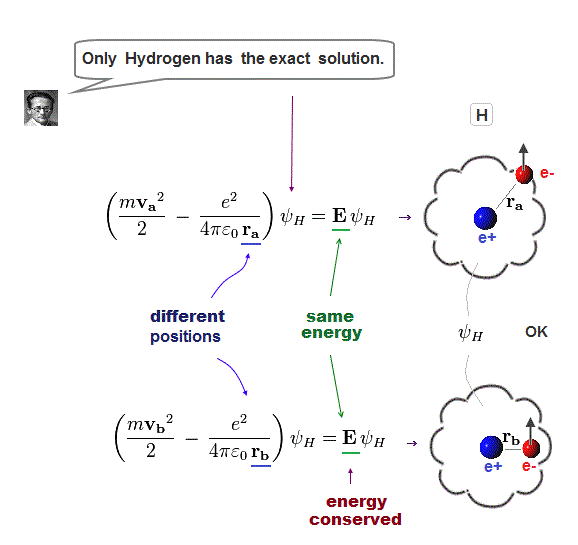
Only Schrödinger equation for one-electron hydrogen atom is solvable, able to give the exact atomic energy that agrees with the realistic Bohr's atomic model ( this-p.21-last, this-p.12, this-4.133 ) also in fine structure (= Bohr model is stable, as shown in successful Bohr model getting Nobel prize, contrary to fake news, this-last ).
There were No computers to calculate three-body Helium atomic two electrons' orbits in 1920s, which is why realistic Bohr model agreeing with experiments was unreasonably replaced by the problematic quantum mechanical Schrodinger equations whose illegitimate approximate methods can never get true helium wavefunctions conserving the constant energy E, so quantum mechanics is proven wrong, and only Bohr's model's helium is right (= now we can use computers luckily ).
↑ Even this only solvable quantum mechanical hydrogen atomic wavefunction is unrealistic in the zero orbital angular momentum where electrons unstably crash into nuclei ( this-p.21, this-p.12-17 ).
And its paradoxical probability wavefunction (whose shape is strangely unchanged despite electrons with kinetic energy constantly moving ) must be always spreading into infinity (= each electron must exist in all places by unreal parallel worlds ), containing unreal negative kinetic energy ( this-p.8, this-p.5-4th-paragraph ), which must be replaced by real de Broglie wave.
(Fig.2') Quantum mechanical Schrodinger equation is completely useless unable to predict any multi-electron atoms or molecules..

Quantum mechanical Schrödinger equation are unsolvable, unable to predict energies of all multi-electron atoms or molecules ( this-p.21, this-p.6 ).
This-p.1-first says -- Unsolvable Schrodinger equation
"For a system with more than one electron, we can't solve
the Schrödinger Eq. exactly ( this-2nd-last-paragraph )"
This-p.4-1st-paragraph says -- Unsolvable helium
"However,
Scientists have tried for decades to solve this ( helium ) three body problem without
succeeding... e it is impossible to determine the eigenstates of the Helium atom"
Physicists have to artificially choose fake trial wavefunctions called basis set functions out of infinite choices (= any forms of trial wavefunctions are allowed to be chosen, this-4th-paragraph, this-p.9-p.14 ), adjust free parameters for the unsolvable Schrödinger equations that can Not predict any atomic energies.
This-p.6-2nd-paragraph says --
No quantum mechanical prediction
"Another aspect that shows ab initio simulations are Not so ab initio as they claim is the basis set... Quantum mechanical calculations are not capable of actually generating their
own basis sets (= artificially-chonsen fake trial wavefunctions ) that must instead be put in ‘by hand’. Furthermore, they are often selected by reference to
some empirical data (= No quantum mechanical prediction )"
This ( or this )-12-13th-paragraphs say -- Wrong Schrodinger equation
"the Schrödinger equation is far from all-powerful... the Schrödinger equation can't yield an exact description of a helium atom"
Even this approximate method of just choosing fake wavefunction is too time-consuming and impractical ( this-p.6-lower-footnote ).
We should use experimental values from the beginning instead of wasting too much time in useless quantum mechanics (= unable to predict anything ) that just hampers real technology.
↑ In fact, we can choose fake helium wavefunctions that can give wrong energy that is lower than the helium's true ground-state lowest energy, which disproves quantum mechanics and its approximate variational methods.
These artificially-chosen (fake) trial wavefunctions or basis set functions for unsolvable Schrödinger equations are unreal, lacking physical meaning ( this-last-paragraph, this-p.1-16.3 ), which can Not give real atomic shapes, hence, hampering nano-technology by relying on fictional overhyped quantum quasiparticle model.
↑ It is said that physicists have to choose fake trial wavefunctions or basis sets consisting of infinite terms ( this-p.8-last-paragraph, this-p.22 ) and infinite free parameters to find the true wavefunctions ( this-p.2-left-3rd-paragraph, this-p.5 ).
↑ Quantum mechanical approximate (variational) methods for unsolvable Schrodinger equations have to find the lowest energy states out of infinite free choices or infinite forms of fake trial wavefunctions (= this-4~5th-paragraphs, this-p.3-lower, this-p.7-8-caveat ) consisting of infinite terms and parameters, which calculations need infinite time, which means predicting true wavefunctions and energies in quantum mechanical Schrodinger equations is impossible forever.
This-p.9-last says Useless infinite terms
"Unfortunately, complete basis sets tend to have an infinite number
of functions and are therefore Not practical for calculations."
This-p.2-right-upper says -- Impractical wavefunction
"However to obtain
exact value of wavefunction b in Equation 2 should
go to infinity (∞) which means that we should
include infinitely many basis functions in our basis
set. Unfortunately this is computationally impractical"
Actually, no matter how many terms and parameters are used, we can never find true atomic wavefunctions for the unsolvable Schrödinger equations, which fact
We can show that it is impossible to find exact atomic wavefunctions (= finding the exact wavefunctions giving the constant conserved total energy E summing electrons' kinetic and potential energies in any positions is impossible ) for any multi-electron Schrödinger equations (or H+ molecular ion ), so quantum mechanics was officially proven wrong.
We should simply use experimental values such as atomic energies and shape instead of wasting too much time in useless time-consuming quantum mechanics that cannot predict anything.
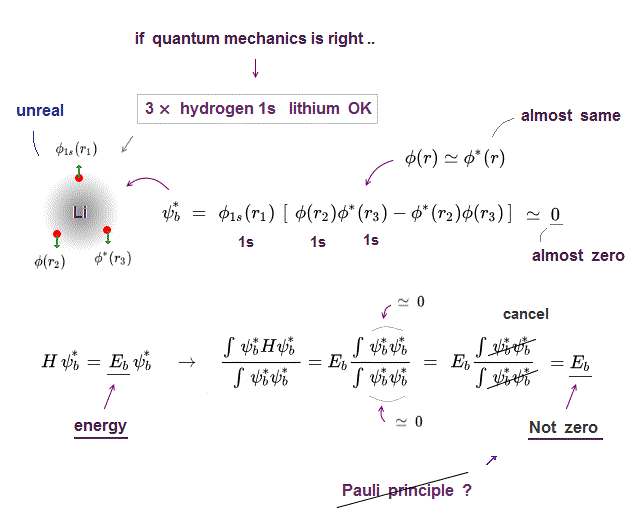
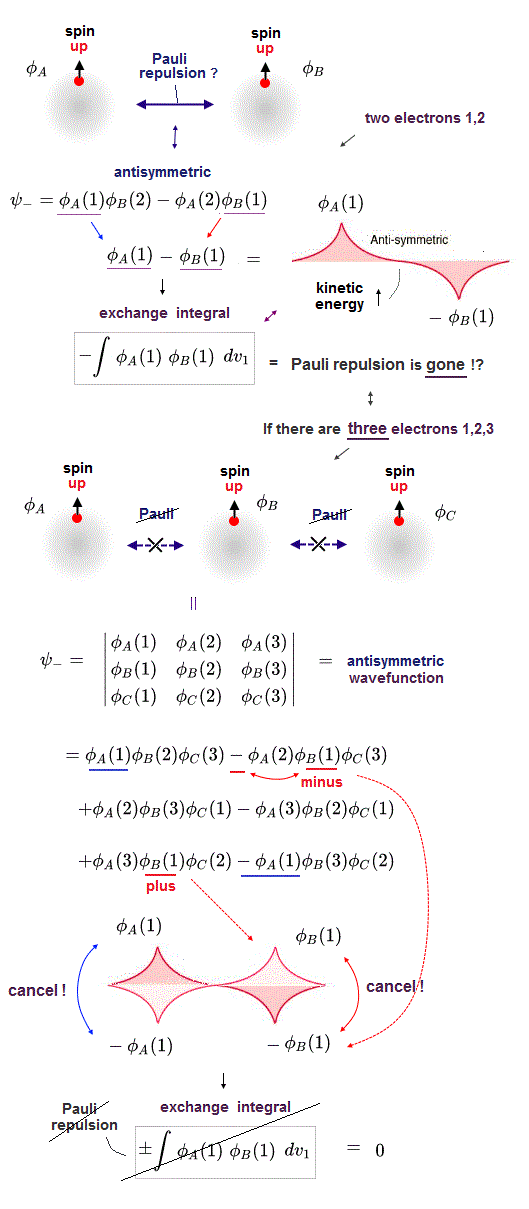
(Fig.P) Electron spin (= 0.0001 eV ) is too weak to explain Pauli exclusion repulsion (= 30 eV ). → Unreal antisymmetric wavefunction or exchange energy was created.
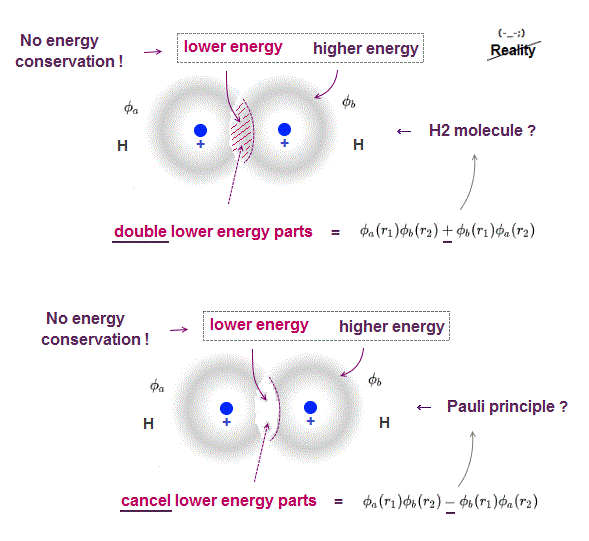
Quantum mechanical Pauli principle baselessly claims that two electrons with the same spin cannot enter the same orbital, which may explain why the 3rd-electron of lithium (= Li ) has to be excluded from the inner 1s to the outer 2s orbital, by overcoming the strong Coulomb attraction.
But the electron spin is unreal, and the spin magnetic dipole-dipole energy between two electron spins is too weak (= 0.0001 eV, this-p.14-p.17, this-p.3-p.4 ), which is unable to explain this strong Pauli exclusion repulsion (= ~20eV, this-2nd~3rd-paragraphs ) overcoming Coulomb force attraction ( this-p.7 ).
And the average magnetic energy of two electron spin up-up (or up-down ) becomes zero in all spaces, so the (unreal) electron spin has nothing to do with Pauli principle.
Electron spin is unreal, cannot explain strong Pauli exclusion principle energy.
So quantum mechanics uses unphysical antisymmetric wavefunction artificially generating exchange energy lacking real exchange force, which increases pseudo-kinetic energy to explain Pauli principle.
This-p.6-2nd-last~last-paragaphs say -- No electron spin
"The magnetic dipolar interaction energy of two ( electron spin ) moments m... This interaction is evidently far too
weak.
Instead, we have to turn back to quantum mechanics and the Pauli exclusion principle which forbids two electrons from occupying exactly the same
state....
"..This is known as the exchange interaction, and although it affects how magnetic moments align, it is fundamentally an electrostatic interaction (= irrelevant to electron spin's magnet ) because it arises from the electrostatic forces (= actually, this unphysical Pauli principle's exchange energy is caused by pseudo-kinetic energy without real force, this-p.9-10 ).. Unlike the ( electron spin's magnetic ) dipolar interaction, the energy of the exchange interaction can be large.
So the quantum mechanical electron spin disagreeing with the actual strong Pauli principle (and ferromagnet, singlet-triplet energy splitting ) has to be replaced by the real electron's orbit with the same Bohr magneton magnetic moment, and the unreal Pauli exchange energy lacking real force or force carriers ( this-p.9-upper, this-p.5-upper ) must be replaced by real de Broglie wave destructive interference.
This-(4) says -- No exchange force
"The exchange interaction is sometimes called the exchange energy or exchange force. However, it is Not a true energy or force."
This-p.2-(c) says -- Unreal exchange force
"Anti-symmetrizing a wavefunction actually pushes the particles
apart, while symmetrization pulls them closer together. This effect actually behaves like an effective force /
pseudo-force, and is sometimes called the exchange force (= unreal pseudo-force )"
(Fig.3'') Quantum mechanical Pauli principle requires each electron to exist in all atoms by illusory parallel universes
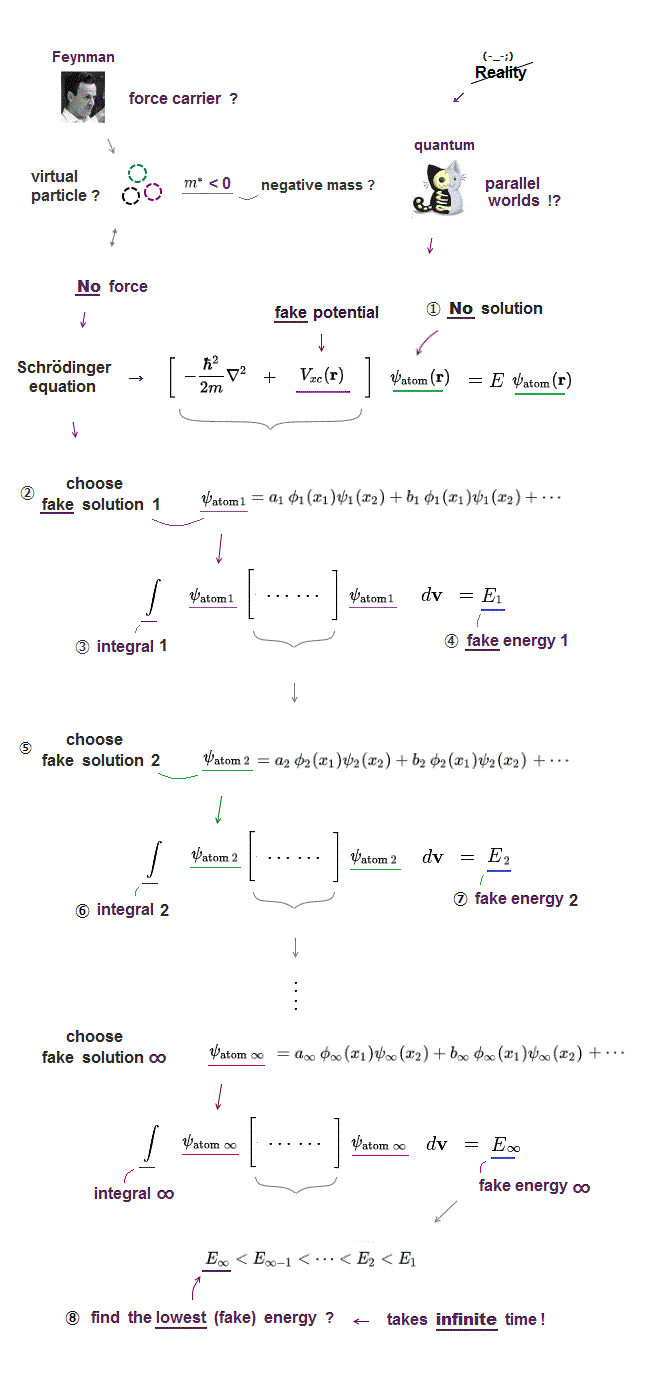
Quantum mechanical Schrödinger equations for multi-electron atoms are unsolvable, useless.
They just integrate the artificially-chosen fake wavefunctions with the Schrödinger energy equation H to obtain the (fake) average total energy E instead of solving the unsolvable Schrödinger equations in the quantum approximate variational or Hartree-Fock methods.
Instead of the unreal electron spin disagreeing with Pauli principle, quantum mechanics demands these artificially-chosen fake wavefunctions (= artificially-choosing means quantum mechanics can Not predict Pauli principle's energy ) must take the form of unphysical abstract antisymmetric wavefunction or Slater determinants ( this-p.13-p.36 ).
In these artificially-chosen unphysical antisymmetric wavefunctions, exchanging any two electrons flips the sign of the whole wavefunction to satisfy Pauli principle (= when two wavefunctions are the same, the whole antisymmetric wavefunction becomes zero ) giving unphysical exchange energy integral ( this-p.11 ) based on pseudo-kinetic energy change lacking real Pauli repulsive exchange force ( this-p.9-upper, this-p.4,p.6 ).
This unphysical Pauli principle's antisymmetric wavefunction requires every single electron to unrealistically exist in all different atoms ( this-p.11 ), which forbids each atom from having shape or boundary, which hampers science.
Quantum mechanics, which just artificially chooses fake antisymmetric wavefunctions for unsolvable multi-electron Schrodinger equations and fake exchange-correlation energy functionals for its one-pseudo-electron DFT approximation, can Not predict any spin or the unphysical exchange energy such as Pauli principle, ferromagnet, singlet-triplet-exchagne-energy splitting, spintronics.
(Fig.S) Quantum mechanical methods for calculating (fake) atomic energy are impractical, too time-consuming.
After choosing fake antisymmetric wavefunctions for unsolvable Schrodinger equations, they repeatedly differentiate these whole Schrödinger energy equations with respect to coefficient parameters (= c ) of the artificially-chosen wavefunctions (= lacking real atomic shape ) to get the coefficient parameters giving the lowest energies (within the chosen wavefunctions) in this approximate quantum variational method ( this-p.21-24, this or this-p.12-20 ).
↑ This unrealistically time-consuming quantum mechanical method is called self-consistent field (= SCF, this-p.53 ) theory where physicists repeatedly choose (fake) wavefunctions, differentiate them with coefficient parameters (= c ), update them until those parameters of the chosen wavefunctions converge to some fixed values ( this-p.10-14, this-p.6-8 ), which often fail to converge.
As a result, quantum mechanical Schrödinger equations are unsolvable, useless, unable to predict any atomic energies ( this-p.1-last ).
So we should stop wasting time in using this impractical quantum mechanical methods, and instead should use experimental values from the beginning.
Even the approximate quantum mechanical methods of just choosing fake wavefunctions, integrating them and repeatedly updating parameters (= SCF ) are impractical, too time-consuming to use for applied science ( this-p.14-last, this-2.22, this-p.2-left-3rd-paragraph, this-p.7 ), hampering technological development.
This unphysical useless quantum mechanical probability wavefunction or superposition originates from paradoxical Einstein relativistic theory.
(Fig.3) Multi-electron Schrodinger equations are useless, too time-consuming → one pseudo-electron DFT or unreal quasiparticle model
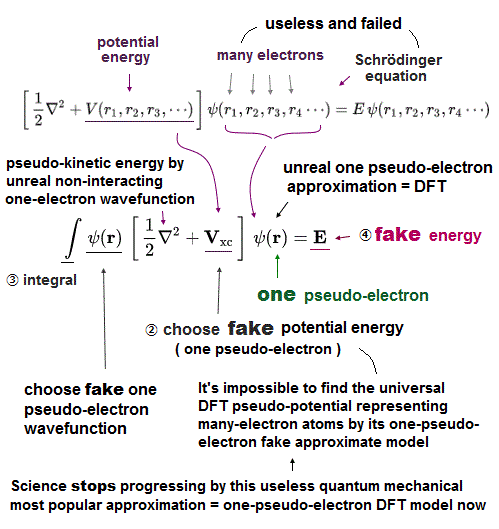
Quantum mechanical Schrödinger equations are unsolvable, unable to predict any multi-electron atoms.
For unsolvable multi-electron Schrödinger equations, approximate quantum mechanical methods have to choose fake trial wavefunctions or bases sets, integrate them and adjust many free parameters to get fake average total energy E, which approximate methods are also impractical, too time-consuming ( this-p.2-3, this-p.11, this-p.14-last ).
Instead of the useless Schrödinger equations, quantum mechanics has to approximately (= illegitimately ) treat multi-electron molecules or material as fictional quasiparticle with fake mass and charge or one-pseudo-electron model called density functional theory (= DFT or Kohn-Sham theory, this-p.18 ) which approximate DFT model has No physical meaning ( this-last, this-p.2-1st~2nd-paragraphs ).
↑ Unreal quantum mechanical Pauli principle's antisymmetric exchange wavefunctions requiring any single electron to exist in all atoms cause this one-fake-electron DFT approximation.
This-p.4-3rd-paragraph says -- Useless Schrödinger
"direct solution of the Schrödinger
equation is Not currently feasible for systems of interest in condensed matter science –
this is a major motivation for the development and use of density functional theory (= DFT )"
This-p.31 says -- Fake one-electron DFT
"the KS (= Kohn-Sham or DFT ) equation ( one-particle equation !! )"
"(quasi)particle states.. fictitious states ( this-p.5-upper )"
This-p.4-1st,4th-paragraphs say -- Unreal quasiparticle
"For solids, we have 1026 electron states. Analytic solution becomes impossible."
"of non-interacting (fictional) quasiparticles.. it can be
recast to give a set of one-particle (= one-electron-DFT ) equations"
This present mainstream quantum mechanical methods or DFT treating materials and molecules as fictional quasiparticle models with unreal ( negative ) effective masses and fractional charges clearly prevent clarifying real microscopic atomic mechanisms and hamper scientific development, which inconvenient fact is hidden by overhyped fake news.
↑ Instead of the useless Schrodinger equations, this current mainstream quantum mechanical approximate ( unreal ) density functional theory (= DFT ) is dominant and used in almost all fields of semiconductors, superconductors, unreal quasiparticles, solar cells, spintronics (= quantum mechanics relying on artificial choice cannot predict any of these physical phenomena, this-p.20-right ) atomic force microscopes, even part of chemistry and biology, hampering nano-technology.
Quantum mechanical multi-electron Schrödinger equations are unsolvable, useless, too time-consuming to choose (fake) wavefunctions out of infinite choices and free parameters to seemingly get the average total energy
So quantum mechanics has to rely on the illegitimate mainstream approximation DFT treating multi-electron molecules as one pseudo-electron or quasiparticle model, which has to artificially choose pseudo-potential or fake exchange-correlation-energy (= XC functional, this-p.2, this-p.8 ) and fake wavefunctions (= basis set ).
↑ Choosing free forms of pseudo-potentials, exchange-correlation energy functionals and wavefunctions means the mainstream quantum mechanical DFT is unable to predict any atomic energies ( this-p.3-5,p.9, this-3rd-paragraph, this-p.2(or p.1)-left, this-p.17-last, this-p.7-lower~p.8 ).
↑ DFT, which cannot predict any physical values, is empirical, fake ab-initio theory ( this-.23-lower, this-p.11-left-1st-paragraph, this-p.21-5.2 ).
This-p.1-introduction-1st-paragraph says -- No prediction
"The precision and reliability of DFT
computations are greatly influenced by the choice of exchange–correlation functional ( this-p.7 )"
This-p.3-right-2nd-paragraph says -- DFT is wrong
"density functional
theory methods in their typically applied forms are Not free
of approximations and therefore, even choosing a robust
functional does Not guarantee perfect results"
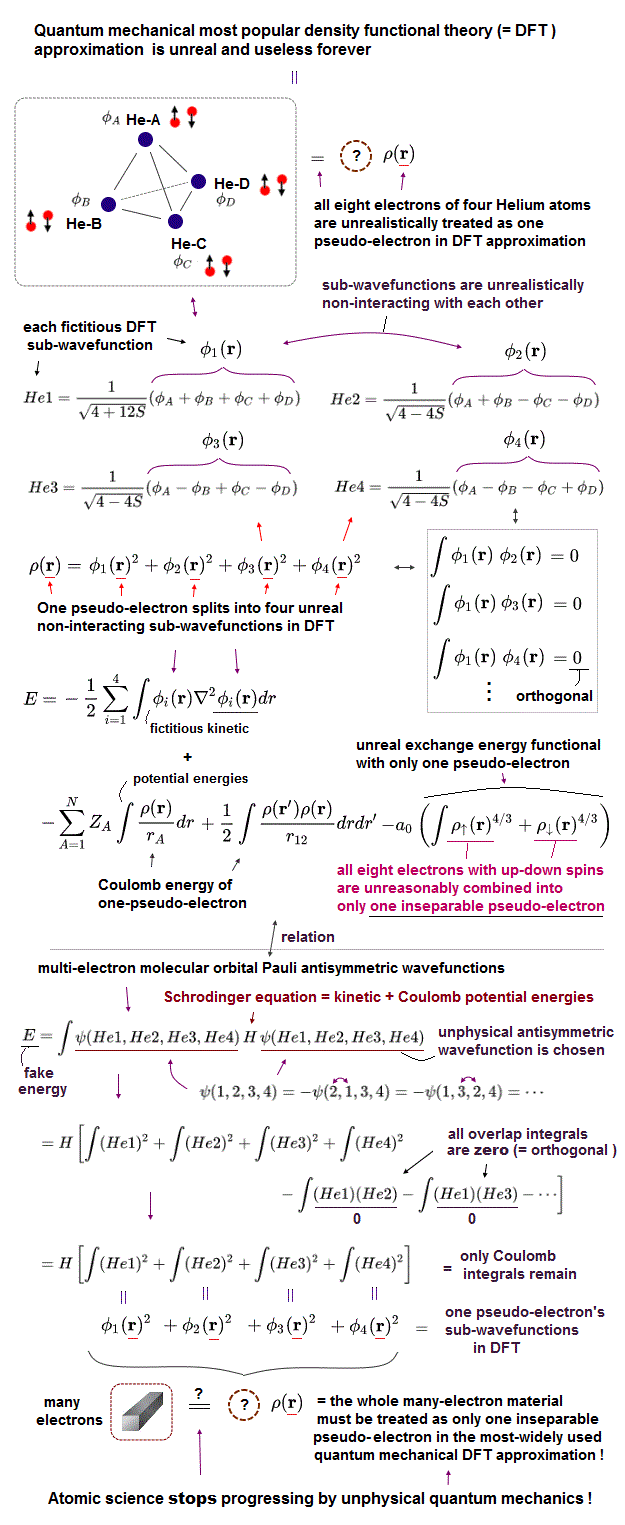
(Fig.3') Today's only mainstream quantum mechanical approximation = DFT just choosing fake pseudo-potentials and one pseudo-electron density is useless.

Even this quantum mechanical (mainstream) approximation DFT is impractical and too time-consuming ( this-p.1-left-1st-paragraph, this-p.1-introduction-1st-paragraph, this-p.5-abstract-2nd-paragraph, ), hampering all the present technologies, medicine, computers.
This-p.10-last-paragraph says -- Useless DFT
"The effectiveness of DFT is highly dependent on the
choice of exchange-correlation functional, which may not accurately capture all correlation effects,.. the calculations of exchange-correlation
energies can still be demanding (= DFT choosing fake exchange energy functional is useless,
time-consuming, unable to predict any atomic energies )"
After integrating the chosen one-pseudo-electron wavefunctions with DFT energy equation, they differentiate them with respect to the coefficient parameter (= c ) to get the coefficients giving the lowest energy E within the chosen wavefunction.
↑ This extremely time-consuming integration and differentiation of the chosen wavefunction must be repeated many times until the coefficient parameters converge to some values, which is called self-consistent field (= SCF ) iteration ( this-p.9, this-p.23, this-p.12~, this-p.11 ).
It is very difficult to get the converged coefficient values, so this DFT based on the time-consuming SCF, which often fail to get the converged energy ( this-p.1-introduction-2nd-paragraph, this-p.1-last-paragraph ), is illegitimate.
Quantum mechanics and DFT must artificially choose fake electron's wavefunctions called "basis set functions ( this-p.10~ )" and initial atomic coordinates to estimate (= Not predict ) some molecular energies, bond lengths, angles in geometry optimization.
This-1st paragraph, & points-to-remember say -- No prediction
"Geometrical optimization,.. is the process by which the geometry of a molecule is adjusted to find a structure with the lowest possible energy."
"Different initial geometries may lead to different optimized structures."
"The chosen quantum mechanical method and basis set (= fake chosen wavefunction ) play significant roles in the accuracy and reliability of the optimized geometry."
This-p.3-lower says -- Chosen fake wavefunction
"Due to the many basis sets and optimization methods, it is very difficult to find the
optimal approach for scientific calculations. The choice of basis set (= chosen fake wavefunction ) for chemical calculations
can have a major impact on the quality of the results,"
This-p.16-1st-paragraph says -- No true lowest energy
"It is important to recognize that this procedure will not necessarily find the global minimum, i.e. the geometry with the lowest
energy. By its nature, a successive search for a minimum finds a local
minimum" ← geometry optimization cannot find the lowest-energy ground state of molecules.
↑ Dependence on artificial choice of basis set (atomic) functions and initial molecular structures means quantum mechanics and DFT are unable to predict any molecular energies or structures.
Integrals of DFT exchange-energy potential can Not be analytically conducted, which must be numerically or approximately calculated ( this-p.4(or p.3)-1st-paragraph ) by dividing space into smaller grids (= 10000 ~ million grids per atom, which grid numbers are free parameters, this-11.9-3rd-paragarph, this-p.2-left ).
This-1st-paragraph says -- No analytical solution
"In practical DFT calculations, the forms of the approximate exchange-correlation functionals used are quite complicated, such that the required integrals involving the functionals generally cannot be evaluated analytically"
This DFT numerical integration takes too much time ( this-lower-10 ), and often causes errors ( this-p.1-left, abstract, this-p.53-54 ).
(Fig.P) Quantum mechanical mainstream DFT is too time-consuming to express energies in different atomic positions
Like the impractical Schrödinger equation, this quantum mainstream approximation DFT is impractical, too time-consuming to use for any technology such as computer transistor, medicine and nanotechnology, hampering making useful multi-probe atomic force microscopes.
When examining the interaction between two molecules (= called geometry optimization ), quantum mechanics or DFT has to calculate fake energies E in many different atomic positions ( this-p.2-1st-paragraph ) and intermolecular distances, by choosing wavefunctions (= ψA, ψB.. = lacking real molecular shape ) whose coefficient parameters (= c ) must be adjusted by the repeated extremely-time-consuming SCF method, which is impractical ( this-p.5-2nd-paragraph, this-p.22 ).
Quantum mechanics or DFT cannot predict atomic positions nor energies.
This-p.4-left-1st,5th-paragraphs, p.4-right-2nd-paragraph say
"The geometry optimisation starts by solving the
Kohn-Sham equations using the SCF method. Then the forces
are calculated and
the positions of the atoms are adjusted by the optimisation algorithm to minimise the energy"
"the calculation of energy derivatives with respect to atomic coordinates for local hybrid functionals is challenging"
"the calculation of forces on one iteration of the geometry optimization requires 6N +1 separate SCF runs where N is the number of atoms (= too many time-consuming SCF iterative calculations are needed in the impractical quantum mechanics or DFT )"
"The numerical differentiation parameter ∆R was chosen empirically (= Not quantum mechanical prediction ), the selection of the optimal parameter for each system under study is intractable for practical applications"
This research took a week ( this-p.5-2nd-paragraph-last ) just to express (pseudo-)potential around small molecules in different positions (= called potential energy surface or PES based on fitting parameters without quantum mechanical prediction, this-p.3-1st-paragraph ) of the atomic force microscope's tip by the unreal DFT ( this-p.7-right-1st-paragraph ). ← impractical.
We should stop using these too time-consuming impractical quantum mechanics and DFT atomic model, and instead, use much simpler practical atomic model with experimentally-measured shape combined with the practical multi-probe atomic force microscope manipulating atoms to cure diseases.
The 2nd-last paragraph of this hyped news (5/23/2025) says
"one step closer (= still useless ) to controlling chemical reactions"
↑ This-paper's p.7-Excited state molecular dynamics used impractical DFT in ab-initio-MD.
p.7-right says " due to SCF or
gradient convergence failures" ← the useless too-time-consuming SCF iterative calculations (= failed ) are still used even now, hampering science.
(Fig.4) Quantum mechanical unrealistic atomic model and fictional parallel universes cannot be used for any useful technology.
Contrary to hypes, quantum mechanics based on fictional parallel universes has been useless for any technologies such as computer's transistors, MRI, atomic clocks, quantum sensors, molecular machines, and medicine.
Because quantum mechanical Schrödinger equations are unsolvable, unable to predict any multi-electron atoms, transistors ( this-6th~10th-paragraphs ), as shown in already-dead Moore's law.
Quantum mechanics has to approximately choose fake trial wavefunctions or basis sets for unsolvable Schrodinger equation, which approximate methods are also too time-consuming and impractical ( this-p.2-3, this-p.11, this-p.4-3rd-paragraph ), unable to predict anything including computer's transistors.
So quantum mechanics has to rely on very rough approximation where the whole molecule and material must be treated as one-pseudo-electron DFT or fictional quasiparticle model ( this-p.18 ) with fake effective mass m* and artificially-chosen fake pseudo-potential in unphysical band model ( this-p.2-5, this-p.14-2nd-paragraph, this-p.5, this-p.6 ) that cannot predict anything including transistors.
↑ Quantum mechanical one fake-electron model is useless, cannot explain actually-moving many electrons in computer transistors.
This (or this )-p.5-left-lower says -- Useless quantum mechanics
"The problem of electrons in a solid – a many-electron problem.... The many-electron problem is impossible to solve exactly... approximation: assume that all the interactions are described by an effective (= fictitious ) potential ( this-p.1, this-p.36,38 )"
Quantum tunneling is irrelevant to the unrealistic negative kinetic energy ( this-1st-paragraph ) claimed by quantum mechanics
As a result, quantum mechanics unable to treat any multi-electron atoms was useless, cannot predict the transistors nor hard disk, contrary to hypes.
Transistors whose electric resistance is changed by the electric current were accidentally discovered by researchers' long experience, trial-and-error approach ( this-lower-trial-and-error approach ) irrelevant to the unphysical quantum mechanics.
This-4th-paragraph says -- No quantum prediction
"but in the exact opposite direction they'd expected."
This-p.16-1st-paragraph says -- Useless quantum mechanics
"there still is No really adequate quantitative theory explaining the working of the point contact transistor...., who observed something that they were not looking for or expecting"
This-p.8-left-2nd-last-paragraph says -- Wrong theory
"Theory also predicted a contact potential difference,.. but the experiments.. failed to verify this point" ← No quantum mechanical prediction
Trial-and-error experiments led to serendipitous discovery of semiconductors whose electric conductance was controlled by small electric current suitable for transistors ( this-4th-paragraph ).
This-middle-Inventing the point-contact transistor-6-7th-paragraphs say
"but unknown to him it had somehow been washed away leaving the gold in direct contact. They were surprised to find that they could still modulate the voltage and current."
"This serendipitous event (= Not quantum mechanical prediction ) pointed them in a different direction"
As a result, "quantum mechanics was needed to develop modern computers !" is fake news.
MRI (= magnetic resonance imaging ) is often said to be caused by quantum mechanics, which is wrong.
We can treat a proton as a classical mechanical particle's spinning, which is different from the unreal electron spin (= a tiny electron must be spinning faster than light to generate the observed magnetic moment ) that is just a classical electron's orbit.
In MRI, they apply the light pulse whose energy (= frequency × ℏ ) is almost equal to the energy difference between up and down (= 2 × ) nuclear spin magnetic moment (= μp = experimental value with No quantum mechanical prediction ) × external magnetic field (= B ) = 2 × μpB ( this-lower, this-p.18-3.17 ) which is equal to the energy equation using geomagnetic ratio (= γ, this-p.3-upper, p.7-upper, this ).
(Fig.Q) Quantum mechanics is useless, describing all physical phenomena as unreal quasiparticles.
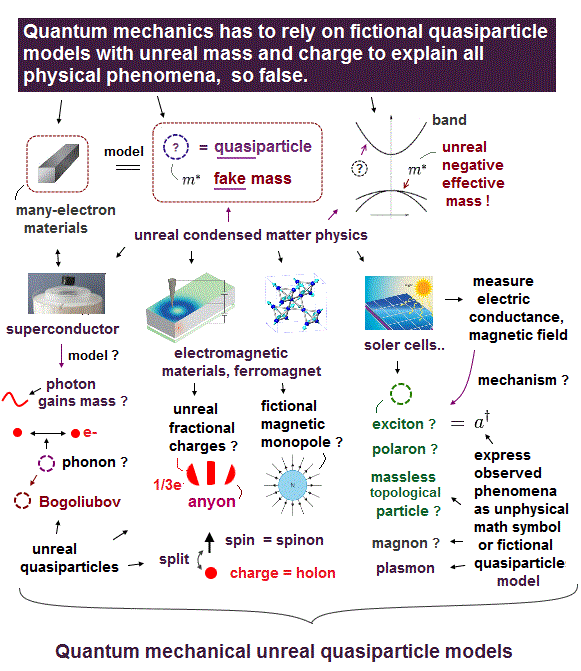
Quantum mechanical Schrödinger equation is useless, unable to get true wavefunctions for any multi-electron atoms or molecules.
So quantum mechanics has to describe any multi-electron materials and physical phenomena only as very old fictional quasiparticle models ( this-summary ) with unreal (negative) mass and charges created in 1930s ( this-p.4-p.6,p.22, this-p.1 ) in the unphysical band model ( this-p.2-p.5, ).
↑ These current mainstream quantum mechanical fictional quasiparticle model lacking real particle shape is one of the main culprits hampering today's nano-technology like one-pseudo-electron DFT model ( this-p.3-p.9 ).
This-1st, 3rd, 6th paragraphs say -- Unreal quasiparticle
"This electron with a (fake) different mass is called an "electron quasiparticle"
"The (fictional) quasiparticle concept is most important in condensed matter physics, since it is one of the few known ways of simplifying the quantum mechanical many-body problem."
"In summary, quasiparticles are a mathematical tool for simplifying the description of solids. They are Not real particles inside the solid ( this-1st-paragraph, this-1st-paragraph )."
This-13th, 18th, 22th paragraphs say -- Fictional quasiparticle
"Remember, quasiparticles are Not real or even theoretical particles"
"it isn't possible to fully and directly describe exactly what's going on inside a single grain of sand! But physicists have an option. They can sometimes use quasiparticle dynamics to simplify such complex systems of atoms"
"It's (= fictional quasiparticle ) Not an exact picture of the microscopic behaviour of a system, and for some systems it won't work,"
For example, quantum mechanics describes superconductors by using unreal quasiparticles such as phonons (= sound wave ) and Bogoliubov ( this-2nd-paragraph ) expressed only as unphysical math symbols lacking real particle shape, as shown in fictitious BCS theory.
They describe some electric conductance or resistance in Hall effect as unreal fractional-charge anyons or Majorana quasiparticles that are completely useless for the hyped ( topological) quantum computers.
And quantum mechanics tries to explain some (useless) electromagnetic properties of materials as an unbreakable electron splitting into unreal spinon (= spin ? ), holon (= charge ? ), orbiton (= orbit ? this-p.5-p.11 ) quasiparticles or fictional magnetic monopole.
Quantum mechanics tries to express solar cells and materials transiently excited by light as unreal exciton ( this-6th-paragraph ), polariton, magnon-spin-wave, skyrmion quasiparticles that are useless, hampering technology, contrary to hyped news.
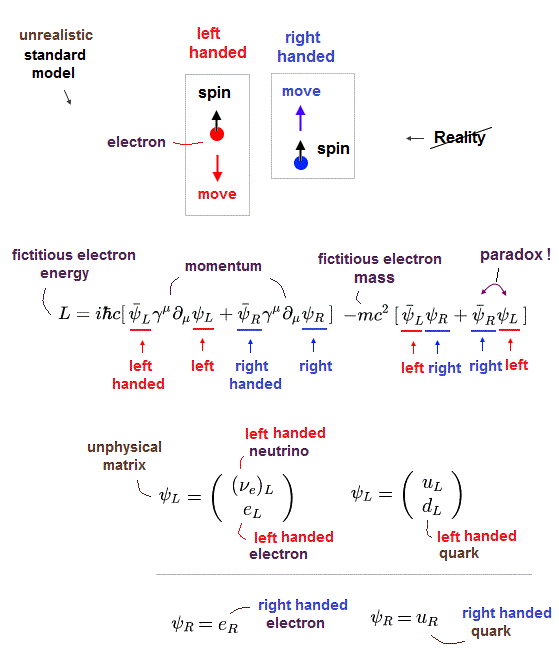
Electrons ejected from materials by light in ARPES are wrongly treated as fictional massless Dirac or Weyl fermion quasiparticles (= allegedly moving slower than light, this-p.1-abstract ) which contradicts Einstein relativity.
Overhyped fake news hides these useless quantum mechanical quasiparticle models.
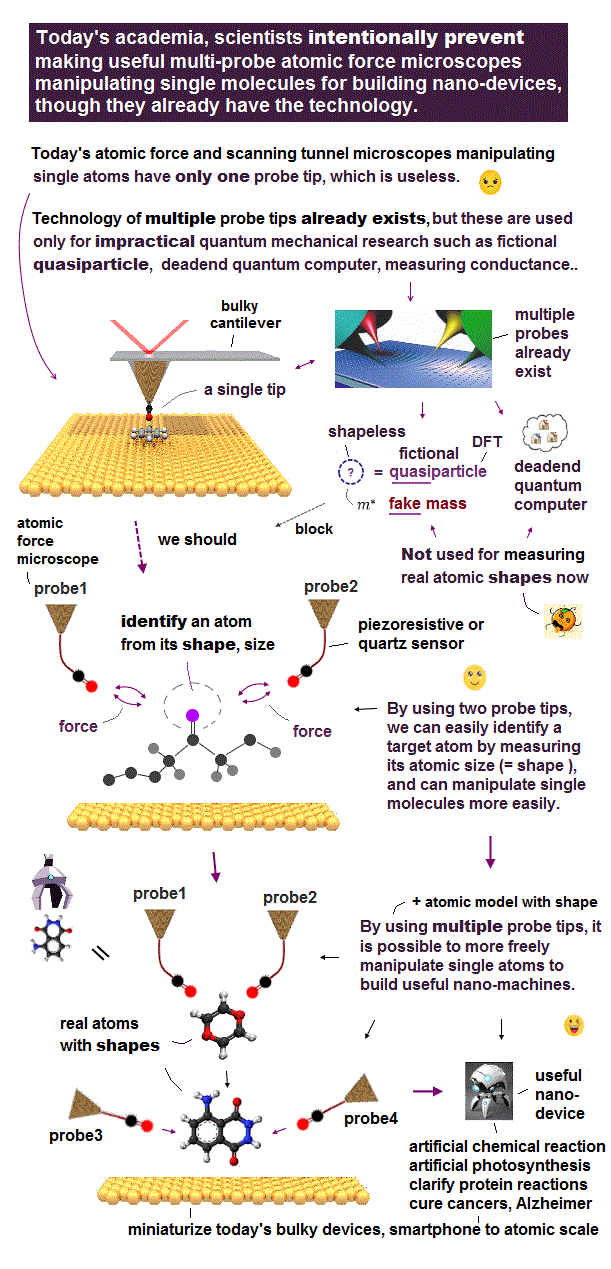
(Fig.5) Atomic force microscope manipulating single atoms still has only one useless probe tip that can see only flat molecules.
Atomic force microscopes (= AFM ) manipulating single atoms were invented in 1980s.
Unlike today's X-ray crystallography and cryogenic electron microscopes with bad (= non-atomic ) resolution, this atomic force microscope is the only tool to directly see single atoms with better sub-atomic resolution, and manipulate dynamical molecules ( this-p.8-right-2. ).
But surprisingly, this atomic force microscope still has only one probe tip. ← No progress for 40 years.
Today's useless atomic force microscope with only one probe can see only flat static molecules like benzene ( this-lower-challenges, this or this-p.12-3D molecules ) , or only upper parts of molecules ( this-p.4~p.5, this-p.2 )
↑ If we try to tilt the probe tip ( this-p.4 ) and push it against the side of the target molecule to see the atoms on the side, this molecule is unstably moved to the other side or rotated, pushed by the probe tip.
Atomic force microscopes are deadend, stuck in only one useless probe tip that can see only flat molecules for 40 years. ← Our nano-technology has stopped progressing.
As a result, today's (useless) atomic force microscopes are mainly used to vaguely see objects and cells which are far bigger than single atoms by using dull probe tips with bad (= non-atomic ) resolution, especially in biological researches.
↑ So the atomic force microscope technology is regressing, no longer seeing single atoms.
Observing 3-dimensional molecules needs multi-probe atomic force microscopes where one probe tip needs to press and fix the target molecule, while another probe tip touches and observes atomic structures of the molecule from various directions by rotating the molecule.
↑ The target molecule pushed by one probe may be rotated, which rotated molecule must be treated like a real object with rotatable shape.
But the current unphysical quantum mechanics must treat the whole target molecule and probe tips as only one-electron DFT or fictional quasiparticle model lacking real atomic or molecular shape.
↑ This quantum mechanical unreal shapeless atomic model (= wavefunction ) cannot describe the actual molecule with shape rotated around some rotating center, pushed by the probe tip (= we cannot define the rotating center in the shapeless quantum mechanical DFT's one-pseudo-electron model ).
↑ Instead of treating a molecule as a real rotatable object with definite shape, quantum mechanics (= and molecular dynamics MD ) has to move each shapeless atom by artificially-chosen (force field) fictitious potential based on one-pseudo-electron DFT model at short time intervals, which is too time-consuming and impractical.
So multi-probe atomic force microscopes combined with real atomic or molecular model with shape are useful for clarifying real 3-dimennsional atomic structures of proteins and curing diseases, which makes today's unreal quantum mechanical shapeless model unnecessary and obsolete.
To protect this current old useless quantum mechanical shapeless atomic model, academia intentionally prevents developing useful multi-probe atomic force microscopes for a long time, though humans already have the technology.
It is extremely strange and unnatural that researchers do Not even try to make useful multi-probe atomic force microscopes manipulating single atoms (= though this is possible in today's technology ), which is in stark contrast to a lot of money wasted only on hopeless deadend quantum computers and information.
↑ We can clarify any 3-dimenisional big protein atomic structures by gradually breaking the target proteins through artificial chemical breaking ( this or this-p.17 ) in experiments.
(Fig.5') Quantum mechanical unreal shapeless atomic model is just a stopgap wrong theory until invention of computers and atomic force microscopes clarifying actual multi-electron atoms by real atomic model with shape.
It is known that No theories including unreal quantum mechanics can solve three-body problems nor multi-electron Schrödinger equations ( this-p.8-Note ).
Knowing multi-electron atomic behavior such as helium needs modern computers and actual experimental observation of single atoms by atomic force microscopes which did Not exist in 1920s when physicists had No choice but to create the stopgap unreal quantum mechanical theory despite fierce opposition.
In 1920s, there were No computers to precisely calculate real multi-electron atomic model such as Bohr model's helium (= now is possible ).
↑ Due to this lack of computers in 1920s, physicists had No choice but to rely on unreal quantum mechanical atomic model with fantasy parallel universes.
Quantum mechanics can only choose fake wavefunctions for unsolvable multi-electron Schrödinger equations, which cannot predict any multi-electron atomic energies.
↑ The chosen (fake) trial wavefunctions can take any forms with infinite terms and free parameters out of infinite choices ( this-4th-paragraph, this-p.8-last-paragraph ), so it is impossible to find solution wavefunctions that give the lowest ground-state energies out of infinite numbers of trial wavefunction candidates, which takes infinite time.
Unreal quantum mechanical Pauli principle contradicting electron spin needs unphysical exchange energy lacking exchange force ( this-p.9-p.10, this-p.9-upper ), which is expressed by ad-hoc antisymmetric wavefunctions.
↑ This unphysical Pauli antisymmetric wavefunction requires every single electron to exist in all different atoms or orbitals, which forbid each atom to have its shape nor boundary.
Choosing and calculating fake trial wavefunctions for unsolvable multi-electron Schrödinger equations are too time-consuming and impractical ( this-p.2-3 ).
So quantum mechanics has to approximately treat the whole molecules and materials as unreal quasiparticle with fake mass and charge or one fake electron model of density functional theory (= DFT ) lacking real atomic shape ( this-p.2-5 ).
These unreal quasiparticle models with fake mass and charge created as an approximate fictional stopgap theory in old 1930s ( this-p.6 ) when there were No computers to calculate many-body or many-particle phenomena are still used even now as a mainstream physics preventing clarifying true particles' phenomena.
↑ This current mainstream quantum mechanical approximation such as unreal quasiparticle or one-fake-electron DFT model are useless for any technologies and hampering clarifying real atomic mechanisms.
Since the invention of modern computers and atomic force microscopes seeing real single atoms in 1990s, we have had technology for clarifying real atomic mechanisms by tackling many-body problems based on real atomic model with shape.
Precisely knowing real multi-electron atomic behavior (= unsolvable three-body problems ) needs modern computers and actual observation of single atoms by atomic force microscopes to know real atomic shape and properties.
After measuring actual atomic shape and properties by atomic force microscopes, we can easily design useful bigger molecular devices by combining atoms and molecules with known shapes and properties as real parts (= it is impossible to predict or design multiple molecular devices without knowing each atomic shape ).
↑ Explaining stable multi-electron atoms and molecules needs not only computer's simulation of Coulomb force but also real de Broglie wave interference related to real atomic shape (= real Pauli principle ) or stable closed orbits, which have to be experimentally measured by multi-probe atomic force microscopes.
Quantum mechanical unreal probability wavefunction and Einstein relativity that do Not admit real de Broglie wave (= due to unreal zero orbital angular momentum ) are unable to describe real many electron phenomena.
It is definitely impossible that theories or just simple old (Schrödinger) equations alone can solve many-body problems or explain real multi-electron atomic behavior.
So "quantum mechanics and QED could predict many physical values of many-electron atoms !" is a complete lie.
Quantum mechanics has relied on unreal quasiparticle model, exchange energy and one fake electron DFT model with artificially-chosen fake exchange potential and wavefunctions, which cannot predict anything, to wrongly and approximately express multi-electron atomic behavior.
Quantum electrodynamics (= QED ) has to rely on unreal virtual particles with imaginary mass and ad-hoc renormalization to illegitimately remove infinities, which cannot predict any finite values such as Lamb shift.
Now we already have technology for clarifying real multi-electron atomic mechanisms by using computers, multi-probe atomic force microscopes and real (= Not stopgap ) atomic model with shape.
But the current old impractical quantum mechanical shapeless atomic ( stopgap ) model hampers clarifying real atomic mechanisms.
It is extremely hard for academia to dismiss the unrealistic old quantum mechanical shapeless model such as fictional quasiparticle or one-fake-electron DFT model (= mainstream theory for long years ) created just as a stopgap (= wrong) theory when there were No computers, even now.
↑ So to protect the current old impractical quantum mechanical model, the academia (= profiting from the old useless quantum mechanics or paradoxical Relativity ) intentionally prevents developing useful multi-probe atomic force microscope that will make the old quantum mechanical theory unnecessary and obsolete.
The old quantum mechanics had tried to do the impossible thing expressing complicated multi-particle phenomena (= unsolvable many-body problems ) only by the simple old impractical Schrödinger equation.
↑ This reckless quantum mechanical attempts caused many unreal concepts such as parallel-world wavefunctions, extra-dimensions, fictional quasiparticle, one-fake-electron DFT model lacking real atomic shape, which unrealistic atomic model hampers nanotechnology making useful multi-probe atomic force microscopes now.
Quantum mechanics is just a stopgap wrong old theory created in 1920s when there were No computers nor atomic force microscopes.
↑ This current old impractical stopgap quantum mechanical shapeless atomic model with unreal parallel worlds just hampers development of nano-technology by intentionally preventing making useless multi-probe atomic force microscopes.
We need to immediately replace the current impractical "stopgap" quantum mechanics with real atomic model with shape to develop useful nanotechnology or multi-probe atomic force microscopes.
Paul Dirac says -- Stopgap quantum mechanics
"Renormalization (= present quantum field theory ) is just a stop-gap procedure. There must be some fundamental change in our ideas, probably a change just as fundamental as the passage from Bohr's orbit (= real atomic ) theory to quantum mechanics (= meaning we need the drastic fundamental change like from today's wrong quantum mechanics into real Bohr's atomic model )"
↑ To hide the current deadend useless quantum mechanics, an incredible amount of overhyped fake news needs to be spread every day.
(Fig.A) AI, Alphafold are useless, because they are trained on bad experimental protein structures obtained by X-ray crystallography or cryo-electron microscopes that cannot clarify real atomic structures.
The overhyped AI and Alphafold cannot predict proteins, posttranslational modification, biological reactions due to lack of experimental data.
Today's best experimental tools are useless X-ray crystallography (= most proteins cannot be crystallized nor seen ) and cryo-electron microscopes with too bad resolution.
And AI, Alphafold cannot predict any molecular or proteins' motion that must be simulated by very old time-consuming molecular dynamics (= MD ) which also hampers nano-technology.
The 3rd~6th, 3rd-last paragraphs of this site (2025) say
"Notable limitations of AlphaFold 3 are that it provides limited insight into protein dynamics and does Not provide realistic protein folding pathways"
"This (protein's) movement can be explored using molecular dynamics (MD) simulations (= MD is the current only method for simulating molecular or protein's motion )"
"while most MD simulations access timescales of tens to hundreds of nanoseconds, depending on the size of the protein. The timeframe we can routinely simulate is approximately 1000 fold too short to capture conformational changes in proteins" ← MD is too time-consuming, useless.
"Unfortunately, it is unlikely that these kind of calculations will be available" ← still No solutions nor progress.
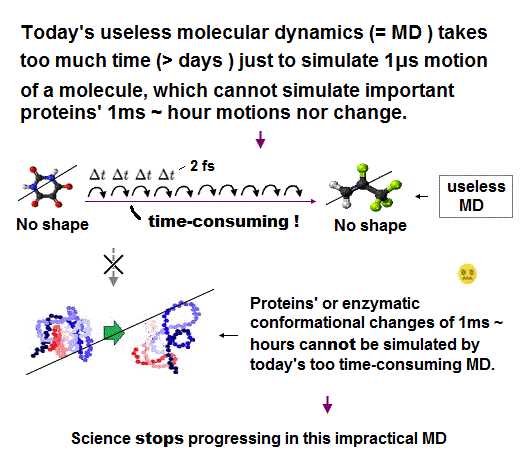
(Fig.6) MD unable to treat a real (rigid) molecule as a real (rigid) object with shape takes too much time to move shapeless atoms at short time intervals (= Δt ) based only on force-field pseudo-potential U.
Today's AI and Alphafold can deal with static (useless) proteins.
Today's only method for simulating molecular or protein's dynamical motion is the very old molecular dynamics (= MD ) which is impractical, too time-consuming to simulate actual proteins' behavior, hampering science.
This-middle-current limitation-2nd-paragraph (2023) says
" time-consuming process includes a one-microsecond ( MD ) simulation of a relatively small system (estimated to be 25,000 atoms) running on 24 processors, which can take several months to complete."
Due to the unphysical quantum mechanical shapeless atomic wavefunction, MD can Not treat each molecule as a real object with shape.
The molecular dynamics (= MD ) has to calculate the positions (= r ) and velocities (= v ) of all atoms at very short time intervals (= each step time interval Δt is 2fs = 2 × 10-15s ) repeatedly many, many times based only on artificially-chosen force field pseudo-potential (= U or V, = space derivative of U is pseudo-force, this-p.2-3, this-p.17-37 ), which takes unrealistically too much time.
Due to the useless quantum mechanics, this force field potential's parameters must be artificially adjusted to fit experimental results (= so MD cannot predict anything ), but often fail ( this-p.5-lower-Limitation of MD simulation ).
Simulating just 1-μs molecular motion needs to repeat all atomic positions' calculations 109 times (= 1μs/1fs = 10-6s/10-15s ), which takes too much time = more than days ( this-p.3-right-1st-paragraph, this-3rd-paragraph ), which can Not simulate actual protein or enzymatic reactions on the time scale of millisecond ~ hours ( this-9th-paragraphs~, this-p.36-38 ).
It is impossible to develop effective drugs by simulating actual proteins' or biological reactions in today's impractical quantum mechanics or extremely-time-consuming MD ( this-modern challenges ).
This-abstract-(in 2025 ) says -- MD is wrong
"molecular-dynamics-simulation models which may Not represent an accurate biological system and thus the predictions will be wrong"
"The computational cost is another drawback"
This-8th-paragraph says -- MD too time-consuming
"One of the main limitations of pharmacophore.. is the complexity of molecular dynamics. This method is computationally-demanding and dependent on the size of the simulated systems, with analysis time ranging from hundreds of nanoseconds to microseconds... which is often too short to analyse protein folding – which can range from milliseconds to seconds. As a result, this can lead to "inadequate sampling" of protein conformations."
This-p.3-left-2nd-paragraph says -- Impractical MD
"atomistic MD calculations using empirical force fields typically use time steps of the order of femtoseconds (i.e. 10-15 seconds), being able to compute few nanoseconds (= ns ) with a personal computer, but far from the millisecond to second timescales of domain motions and allosteric transitions occurring in some enzymes"
Even the latest molecular dynamical (= MD ) researches ( in 2025 ) could simulate just less than 200ns (= 10-7s ) molecular or protein slight motion ( this-p.4-MD-simulation, this-p.4-1st-paragraph, this-p.12-right-Methods ) due to much time required ( this-middle-Benchmarking results ), which is far from time-scale of biological reaction (~ hours ) or cancer formation (~ years ) ← cancer is incurable.
This recent overhyped MD research-V.Resuts, VI discussion (2/19/2025) says today's MD took a day to simulate (only) 0.3μs molecular motion (= too time-consuming ), and this latest research took a day to simulate (still only) 0.1ms metallic atomic motion (= still cannot simulate 1s ~ hours proteins' behavior ) using the empirically-fitted embedded atom method (= EAM, this-p.1, this-p.1-left-last ) with only (fake) short-range pseudo-potential that can Not be used for actual proteins' simulation.
(Fig.M) MD cannot predict when two quantum mechanical shapeless atoms collide.
Due to the unrealistic shapeless quantum mechanical atomic wavefunction, MD can Not predict when two shapeless molecules collide with each other, so each calculation step's time interval (= Δt ) of MD must be extremely short = less than 2fs = 2 × 10-15 second (= which takes too much time to repeat many calculation steps ) to avoid the catastrophic collisions or unreal overlap of two molecules that cause errors.
This-p.10-2~3rd-paragraphs say -- Unreal overlapped atoms
"Too large a time step (= Δt > 2fs ) can cause a molecular dynamics simulation to become unstable (= error ), with the
total energy rapidly increasing with time. This behavior is often colloquially termed "exploding"
and it is caused by devastating atomic collisions that occur when a large time step propagates
the positions of two atoms to be nearly overlapping; the ( Pauli ) repulsive interactions then create a
strong force that propels these atoms apart."
See this-p.37-1st-paragraph, and this-p.27-28.
↑ So the molecular dynamics (= MD ) is impractical, too time-consuming to move each unreal shapeless atom little by little at very short time intervals (= each time step is < 2fs ) many. many times to avoid unrealistic overlap of two shapeless atoms.
If we treat each molecule as a real object with the actual (= experimentally-measured ) shape, we can easily predict when two molecules with shape collide and how they change their motion after collisions (= without unreal overlap of two shapeless atom ) even without the impractically time-consuming MD simulation, like easily predicting billiard ball's trajectory and rigid body's collision (= without MD simulation ).
↑ Actually, we do Not use the extremely time-consuming molecular dynamics nor quantum mechanics to design and build useful dynamical robot machines, cars, planes, rotating gears, all of which have shapes, in our daily life.
↑ all macroscopic objects have their "shapes", which means microscopic atoms composing the macroscopic objects also have their tangible "shapes", which obvious fact is unreasonably ignored by quantum mechanics,
Coarse-grained molecular dynamics (= CGMD ) is one of rough approximate MD methods treating multiple atoms as a fictional pseudo-atom, which is also too time-consuming ( this-p.3-left-coarse grain molecular dynamics ) and unable to clarify actual protein reactions at the atomic level ( this-last, this-p.2-left-2nd-paragraph ).
Artificially-accelerating molecular dynamical methods such as Gaussian accelerated GaMD are also impractical and too time-consuming (= this-p.6 conducted just 2500ns simulation, this-p.3-2.3 says even accelerated GaMD took a day just to simulate 500ns motion = too time-consuming, impractical ).
Ab-initio or first-principle MD (= AIMD, FPMD, Car-Parrinelo CPMD = fake ab-initio unable to predict anything ) based on quantum mechanical DFT pseudo-potential and fictitious electron mass ( this-p.20, using unreal spreading electron's wavefunction ) is much more time-consuming and impractical than the ordinary (= classical ) MD ( this-p.2-left-1st-paragraph, this-p.2-I.introduction, this-p.19-3rd-paragraph ).
Monte-Carlo (= MC ) moving each atom randomly is also too time-consuming ( this-p.2-right-1st-paragraph ), and often unable to give right reactions ( this-p.2-right-last-paragraph ).
This-p.1-left-3rd-paragraph says -- Useless Monte-Carlo
"MC (= Monte-Carlo ) simulations apply random moves to sample the phase
space,.. Therefore, MC moves do Not need to
have any physical meaning.... for complex molecules such moves
can easily lead to steric clashes. A simulation of protein bovine
pancreatic trypsin inhibitor has shown that MD can be as
much as 10 times more efficient than MC (= Monte-Carlo is more time-consuming and impractical than MD )"
(Fig.8) The present useless physics just wastes money and time in fictional concepts that are harmful to not only us but also researchers ruining their careers. ↓

Today's unphysical quantum mechanics based on unreal quasiparticle model has hampered all technologies, medicine, solar cells, spintronics.
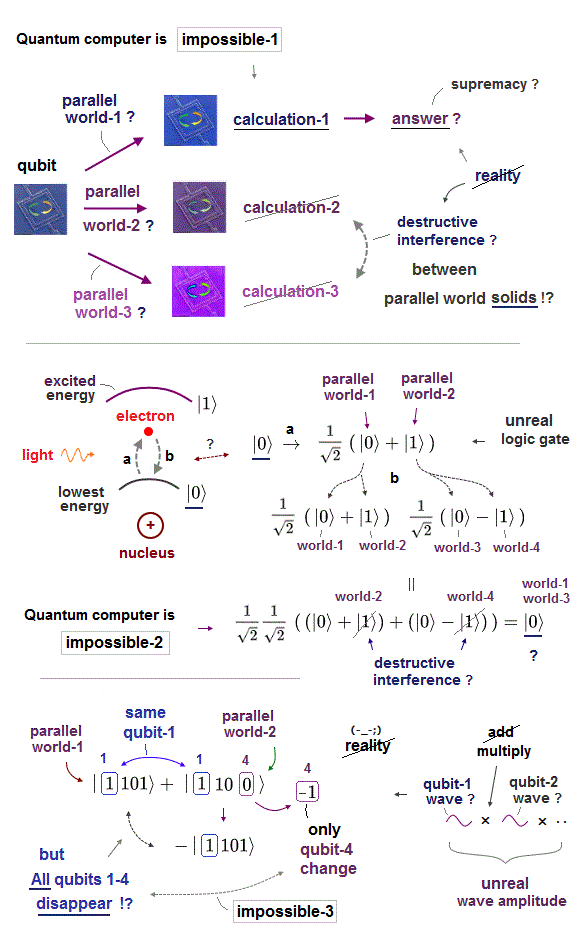
So this deadend physics needed to create fake useless scientific targets such as BigBang, black hole, wormhole, gravitational wave, extra-dimensional unified theory, superluminal entanglement, hopeless hyped quantum computers (= based on fantasy parallel universes ), internet, memory, AI.
Paradoxical relativistic quantum electrodynamics (= QED ) says electromagnetic force is mediated by (unreal) virtual photons with imaginary mass violating Einstein mc2 relation ( this-p.3 this-p.3 ).
This QED and particle physics pursuing only illusory useless particles just give meaningless infinities that must be illegitimately removed by ad-hoc renormalization, which cannot predict any physical values, contrary to hypes.
(Fig.9) Useless quantum mechanics prevents development of all applied science. Cancers, Alzheimer are still incurable. Moore's law is already dead.
In fact, the present science and technology has already been a deadend with No more progress.
Cancers and Alzheimer are still incurable.
Renewable energy such as solar and wind-power generation, electric cars heavily relying on taxpayers' subsidies are still too expensive and too energy-inefficient to replace fossil fuel.
Nuclear fusion technology has stagnated, too.
Moore's law is already dead, and No more progress in computer, smartphone, (hyped) AI technology, quantum computers (= deadend ) is expected.
↑ All these present stalled technologies originate from today's deadend nanotechnology manipulating atoms hampered by the mainstream academia only to protect the old useless quantum mechanical model.
As shown in the industrial revolution, the real science and technology originally made our life convenient and happy.
But the current deadend (deceptive) mainstream science based on the fictional quantum mechanical parallel universes, paradoxical Einstein relativity, extra-dimensional theory of everything just makes our life inconvenient and unhappy by wasting taxpayers' money in still-useless expensive electric cars, gigantic particle colliders, black hole.
The present space industry is also stalled, just wasting money in trying to go to the moon, which was already done more than half a century ago (= No progress ).
And the current (fictional) science just imposed the inconvenient unnecessary restriction such as COVID-19 lockdown causing terrible inflation, mandate of ineffective vaccines, gas stove ban, higher tuition for worthless universities whose impractical science causes job-skill mismatch and truancy..
↑ The current deadend mainstream (pseudo-)science is harmful, Not beneficial for us.
(Fig.10) Today's science is useless except for publishing papers in journals. ↓

The important point is that almost all the current researchers, whether in academic universities or private corporations, waste their time just aiming to publish papers in journals, focusing only on useless pseudo-science instead of really aiming to cure diseases or invent useful machines.
No matter how many times they published papers in prestigious academic journals, cancers and Alzheimer are still incurable, quantum computers are still far from reality (= rather regressing ) due to the journals' impractical pseudo-science.
These (prestigious) academic journals and Nobel prize are used as a means to enable even the impractical pseudo-science such as a dead-alive cat, fictional quasiparticle model, hopeless overhyped quantum computers, paradoxical black holes, extra-dimensions to get taxpayers' money as science research funds.
↑ In order to make today's deadend hopeless (pseudo-)science look 'promising', academia tries to utilize academic journals and prestigious prize as the only (fictitious) holy grail of science and technology. ← The actual science stops progressing behind it.
(Fig.11) Today's unrealistic mainstream science allows universities to skyrocket tuition limitlessly. ↓

Basically, it is impossible for old-fashioned archaic universities with more than 100-year history to maintain the leading scientific positions (= deserving exorbitant tuition ) in the competitive science and technology field by overcoming many for-profit corporations and start-ups.
The trick is they impose the impractical pseudo-science on us as the mainstream science, colluding with academic journals (= worshipped as the holy grail of today's impractical mainstream science by all industries ) and the (overhyping) media to suppress the free new useful scientific idea and hamper new technological innovation in all industries including private corporations and start-ups ( by "brainwashing" them using almost all the media and scientists ).
So the current impractical (unrealistic) mainstream science keeps giving the archaic universities the (pseudo-)scientific leading position and privilege (= by suppressing all industries ), and allows them to skyrocket tuition limitlessly.
↑ Even if you avoid going to universities. you cannot advance science and technology freely under the current old impractical restrictive mainstream science where whether people graduate from (expensive) universities or not becomes the only standard for estimating their academic ability, which unreasonable repressive system keeps giving (pseudo-)scientific privilege to old archaic universities.
Originally, we can learn "science", even if we don't go to so expensive universities. So there is something wrong in the current science and educational system, whose impractical science is used just as political tools, making our life unhappier by causing skill-job mismatch, massive student loan, lower birth rate..
To hide this inconvenient truth of today's deadend science, they spread overhyped fake science news everyday.
And corporations (including spinoffs from universities ) involved in today's impractical science such as the deadend quantum computers try to raise stock price by cilluding with the academia the media, spreading overhyped fake news.
(Fig.12) Quantum mechanics can describe each electron or fictional quasiparticle as a nonphysical abstract math operator (= a†, b†, c† ) with No shape.↓
The important point is that the unphysical quantum mechanics is unable to express any atoms and electrons as real particles with concrete shapes and size.
Quantum mechanics can only describe each particle, electron (= fermion ), photon, quasiparticle as nonphysical math symbols called creation (or annihilation ) operators (= each particle is denoted only as a†, b†, c†.. ) with No concrete physical shapes ( this p.2-5, this p.3-5, this p.7-8, this p.4~6, this p.17 ).
So there is a wide discrepancy between the media's misleadingly colorful fake images of atoms and the unphysical shapeless quantum mechanical atomic models (= each particle is denoted as a nonphysical math symbol ) that are used by physicists in research papers ( this p.2~20, this p.2, this p.2~4 ).
See the wide discrepancy between the science news' false imaginary atomic electron picture that appears to be real and its research paper's abstract description of electrons just as unphysical math operator with No shape (= this p.2~ ).
Compare another science news' misleadingly colorful picture and its research's nonphysical electron math symbols with no concrete figure ( this-p.2 ).
↑ Compare also this and its research paper p.2-(S1), this and its research paper-p.2-(2), this and its research paper. ← Overhyped science news tries to implant false colorful image into us to make the unphysical quantum mechanics appear to be real.
This unphysical abstract quantum mechanical particle models (= just nonphysical symbols with No shapes ) can Not be utilized as tools to design or build useful molecular devices.
Quantum dot (= QD ) is a very tiny semiconductor material.
Each quantum dot is called "artificial atom" consisting of many (~10000) atoms.
Quantum dot has some discrete energy levels emitting light wave with some wavelengths related to its size, many-body effect and de Broglie wavelength ( this p.3 ) used in realistic atomic model (= Not fantasy quantum mechanics ).
Quantum mechanics just tries to explain the quantum dot by using fictional exciton quasiparticle model with fake effective mass in the band ( this p.2~6, this p.1,3 ), which can Not clarify the true mechanism (= So there is No evidence of quantum mechanics related to quantum dots ).
Quantum dot spin qubit is also useless, irrelevant to (unphysical) electron spin.
(Fig.A) Cloning and expression of the target protein's DNA inserted into virus plasmids by bacterial enzymes such as PCR polymerase, restriction enzymes, ligase. = No (useless) quantum mechanics is used
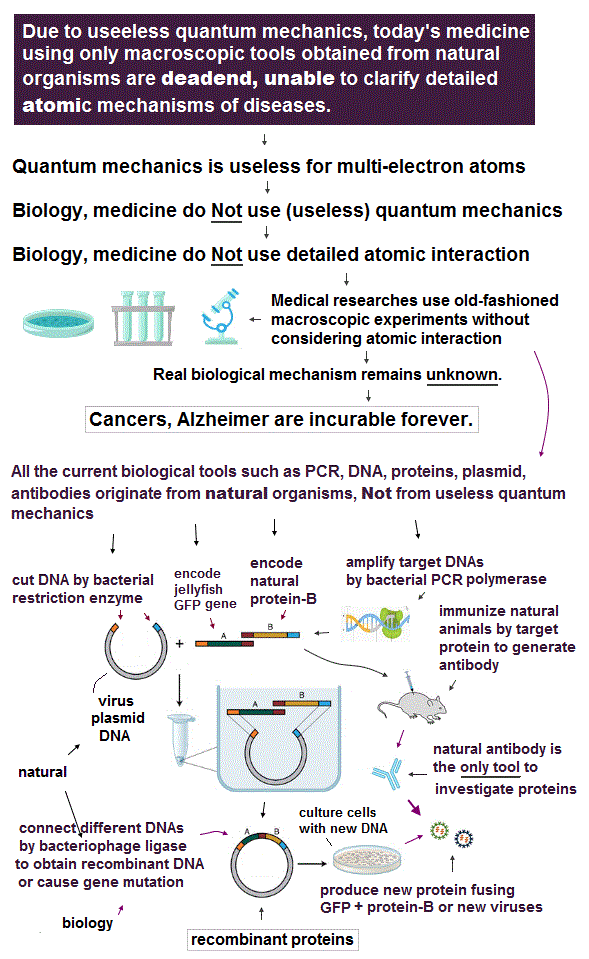
All the current biological and medical research tools such as DNAs, PCR polymerase came from natural organisms, Not from being designed by humans nor useless quantum mechanics that just obstructs medical development and curing diseases.
So humans cannot design or manufacture truly-artificial proteins from scratch for new drugs or medical treatment.
When trying to create 'new' proteins, all researchers can do is just modify or fuse natural proteins by deleting some genes of natural proteins (= called mutagenesis ) or fusing multiple natural proteins into one new recombinant protein by using enzymes and biological tools such as PCR enzymes obtained from natural organisms.
(Fig.M) Today's medical research does Not use (useless) quantum mechanics nor detailed atomic interaction ↓
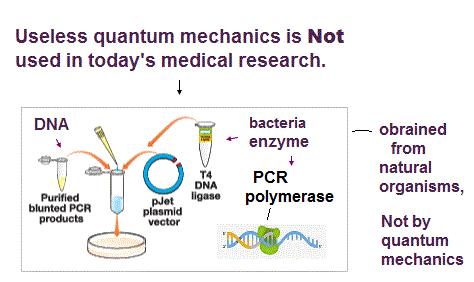
1. obtain DNA genes encoding target natural proteins.
If this target protein is encoded in RNA or mRNA like RNA viruses, change the target RNA into DNA (= called cDNA ) by enzymes called reverse transcriptase that originated from natural retroviruses. → RT-PCR.
2. amplify the target DNA by PCR polymerases which enzymes originated from natural bacteria using primers that can encode restriction enzyme cutting sites and mutations.
3. cut the amplified DNA and plasmid vectors (= originating from bacterial viruses ) that already encode another (natural) protein such as green fluorescent proteins (= GFP originating from jellyfish ) by restriction enzymes (= originating from bacteria ).
4. insert the cut target DNA into the cut plasmid by ligase which enzyme originates from natural organism, too.
5. Infuse cultured cells or bacteria with these plasmid DNAs encoding the target protein (= called transformation or transfection ), and let them produce new recombinant proteins such as the target natural protein joined to GFP from jellyfish.
↑ As shown here, today's biotechnology uses only macroscopic tools originating from natural organisms that do Not clarify atomic mechanisms nor use (useless) quantum mechanics.
(Fig.A) Today's medical research does Not use (useless) quantum mechanics nor detailed atomic interaction
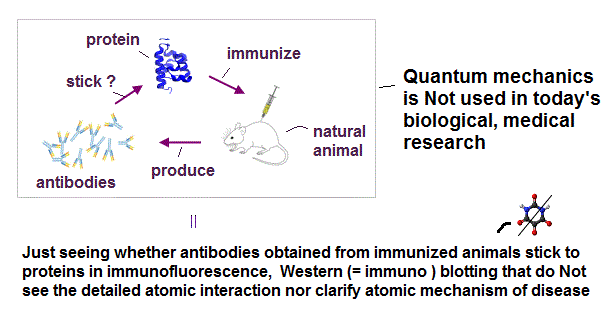
In the current biological and medical researches, the only way for researchers to guess the functions of proteins is ( unreliable ) antibodies obtained from immunizing natural animals. ← Natural immune system is used to produce antibodies that cannot be designed by (useless) quantum mechanics.
They can only vaguely see whether antibodies with fluorescence may stick to target proteins macroscopically without knowing detailed atomic nor molecular mechanisms between proteins and antibodies.
These biological methods using antibodies are immuno-fluorescence, western blotting, immuno-precipitation (= IP ), flow-cytometry, ELISA ( this p.4 ).
It is impossible to know where in the target protein the antibodies stick or how proteins change their functions or conformations after interacting with antibodies, because today's biology does Not consider detailed atomic interaction due to useless quantum mechanics. ← effective drug development is impossible.
Useless basic physics or quantum mechanics prevents medical researchers from utilizing detailed atomic interactions, hence, finding effective drugs and treatments for deadly diseases such as cancers and Alzheimer is impossible.
(Fig.M) Science and technology stall by unphysical quantum mechanical DFT and extremely-time-consuming MD.
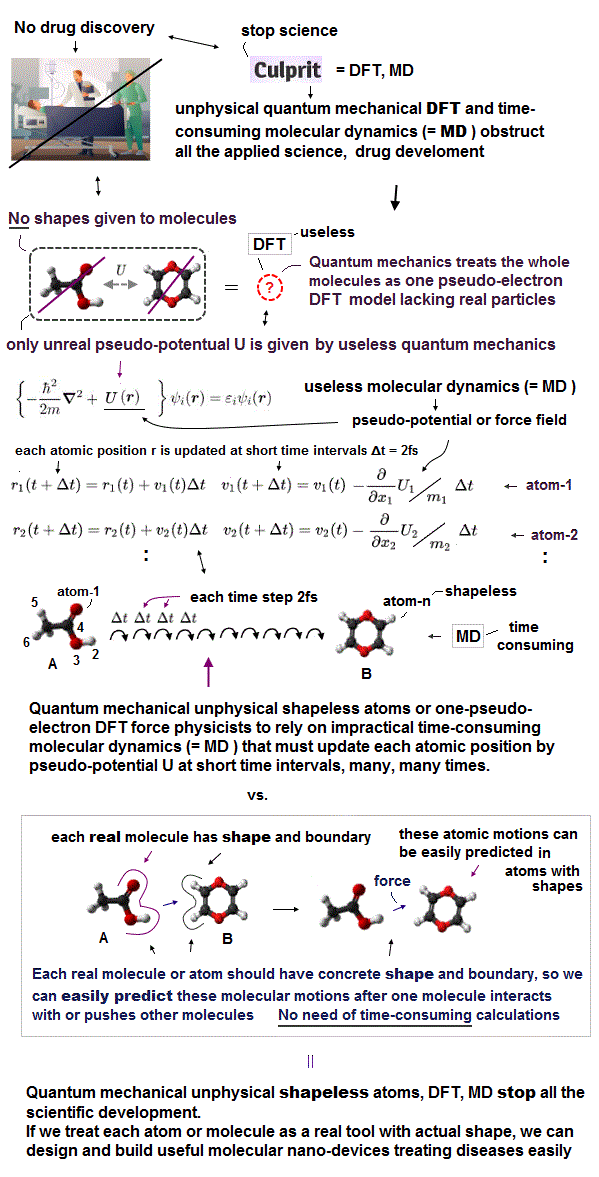
Today's medical research is deadend, unable to clarify atomic mechanism of diseases due to unphysical quantum mechanical shapeless atomic model such as DFT and impractically-time-consuming molecualr dynamics (= MD ).
(Fig.2) Quantum mechanics + Einstein relativity = string theory.
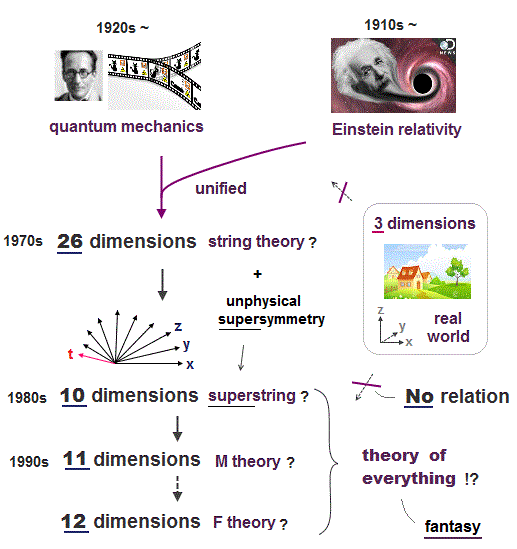
Einstein dream = theory of everything is supposed to unify quantum mechanics and Einstein's theory of relativity.
In 1970s, as the first theory of everything, unrealistic 26-dimensional string theory was invented.
Our real world is 3-dimensional (= x,y,z ), which is incompatible with this extra-dimensional theory of everything.
In 1980s, this fictional 26-dimensional string theory incorporating another fictional theory called supersymmetry turned into new theory of everything called superstring theory which still has 10 extra-dimensions.
In 1990s, this 10-dimensional superstring added one fictitious extra-dimension, and developed into 11-dimensional M theory which is supposed to be the present leading theory of everything.
The latest version of these fantasy unified theories is 12-dimensional "F theory".
As you see, quantum mechanics and Einstein relativity were so unrealistic that their unified theory, a.k.a. theory of everything is also filled with fictional extra-dimensions, parallel worlds, and wrong math (= 1+2+3 .. = -1/12, this 4th paragraph ).
These current so-called mainstream "science" is Not "science" but just illusion which has No relation to real physical phenomena around us.
(Fig.3) Electrons in "s" orbitals with unreal zero orbital angular momentum always crash into nucleus . → unstable
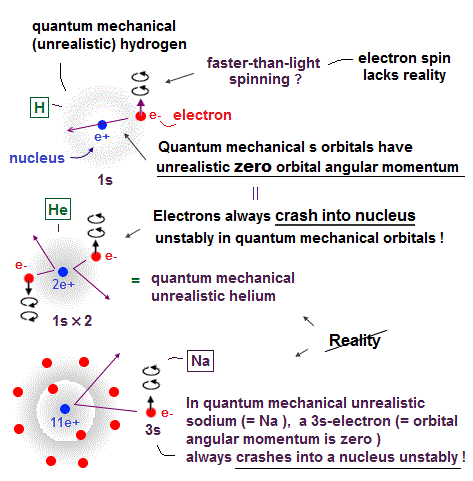
All quantum mechanical atoms are said to have the unrealistic zero orbital angular momentum L = 0 in the s orbital ( this p.9 ).
↑ It means electrons of all atoms such as hydrogens, heliums, sodiums.. with the unrealistic s orbitals must always crash into nuclei in their linear orbital motion (= zero orbital angular momentum ). ← Quantum mechanical electron also must be actually "moving" because of their non-zero kinetic energy
In this unrealistic zero orbital angular momentum or linear motion, electrons experience destructive interference of de Broglie wave (= experimentally-verified ), hence, quantum mechanical atoms become unstable and impossible.
As a result, Bohr's atoms are real without this unreal zero orbital angular momentum (= Stable Bohr's electrons do Not crash into nuclei nor cause destructive intererence of de Broglie wave ) nor electron spin.
Quantum mechanical helium atom consisting only of 2 × 1s states with zero orbital angular momentum is unrealistic.
Because Coulomb repulsion between electrons prevent electrons from doing linear motion or zero orbital angular momentum (= Two electrons of a helium must naturally have orbital angular momentum due to avoiding other electrons )
↑ If the quantum mechanical helium has non-zero-orbital angular momentum, this disagrees with the experimental helium ground-state energy, because the quantum mechanical orbitals always contain kinetic energy in radial direction (= circular motion is impossible in quantum mechanical orbitals due to the unreal probability wavefunction ), which drastically increases kinetic energy by increasing the principal quantum number from n = 1 (= radial ) to n = 2 (= radial + angular momentums ).
Quantum mechanical 2s, 3s, 4s .. states are unreal, because they always contain nodes where electrons' probability is zero ( this-lower ).
↑ Electrons must unrealistically move from one side to the other side through the nodes with zero electron-probability, which is contradictory.
(Fig.4) Schrödinger's 2p radial wavefunction, negative kinetic energy area.
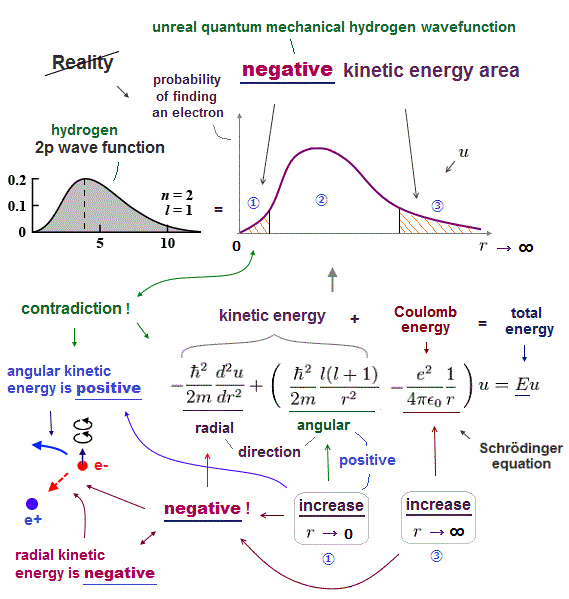
As shown in the upper figure, all the quantum mechanical wavefunctions include the regions of unreal negative kinetic energy, so quantum mechanics is unphysical and false.
For example, in hydrogen's 2p wavefunction with orbital angular momentum L = 1, in the regions closer to the nucleus, kinetic energy in the direction of angular momentum increases to infinity ( = L(L+1)/r2 → ∞ r → 0, this p.3-lower, this p.2-lower ), which must be canceled by the impossible negative kinetic energy in the radial direction.
And in the region far away from the nucleus, Coulomb potential energy is higher than the total energy E, which must be canceled by the impossible negative kinetic energy ( this p.2-3, this-middle-Example 2 ).
Quantum mechanical atomic wavefunctions can never avoid this unreal negative kinetic energy, which fact disproves quantum mechanics.
Quantum mechanical unphysical wavefunctions are said to express probabilities of an electron existing in multiple places and the electron's kinetic energy obeying de Broglie wave relationship.
↑ For each electron to exist in one place, this unphysical quantum mechanical probability wavefunction must be extremely acute and localized in only one place, which increases the electron kinetic energy to unreal infinity due to shorter de Broglie wavelength increasing kinetic energy.
So the unphysical quantum mechanical uncertainty principle insists each electron with finite kinetic energy must always exist in all places simultaneously by using ( fictional ) superposition or parallel universes.
Unlike the textbooks' ordinary explanation, it is to impossible to "measure" each electron's position, because it is impossible for the measured electron to have infinite kinetic energy, which fact disproves quantum mechanics.
(Fig.P) Quantum mechanical probability wavefunction is contradictory, wrong.
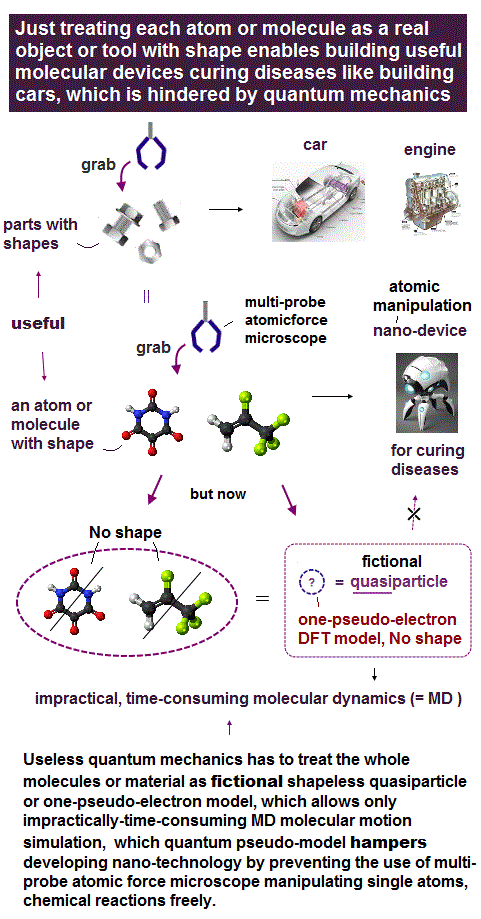
If quantum mechanical probability wavefunctions are true, the wavefunctions change their shapes with time due to electrons with kinetic energy constantly moving and changing their location probability, and the original probability wavefunctions become invalid soon.
In the upper figure, the middle b region has the highest electron's probability at first in hydrogen 1s wavefunction.
But the electron has kinetic energy at b region, so this highest-probability wavefunction soon moves from b to other regions (= toward infinite distance ) like toward "a" farther away from the nucleus.
↑ This means the shape of wavefunction changes and the electron's kinetic energy distribution also changes, because the wavefunction expresses de Broglie wave theory (= de Broglie wavelength inversely proportional to electron's momentum or kinetic energy ).
As a result, the electron's probability wave at "a" region, which has no kinetic energy at first, gets positive kinetic energy (= due to the highest-probability wavefunction in "b" region moving to "a" which is equal to de Broglie wave with positive kinetic energy ), and the electron probability wave can Not stop at "a" due to the newly-acquired positive kinetic energy, hence, flies away into infinity, which cannot give the correct atomic energies.
This contradictory probability wavefunction shows quantum mechanics is wrong, which originates from paradoxical Einstein relativity.
(Fig.P') An electron's probability wavefunction must spread into infinity for an electron to move in one direction, which is impossible.
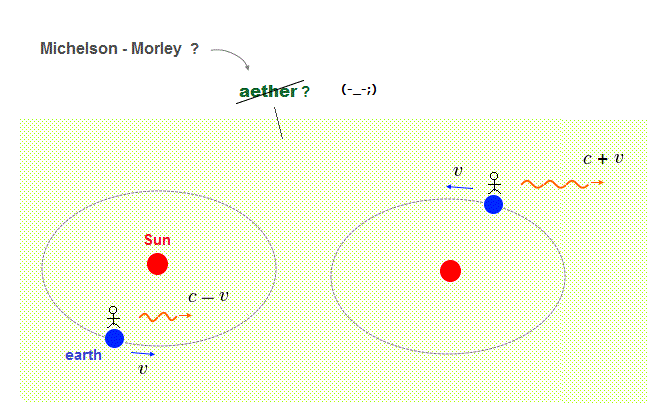
We can easily disprove quantum mechanical probability wavefunction.
In the upper figure, an electron confined in small area in an atom is excited by light to move linearly in x direction.
But for an electron to move in one (= x ) direction, this electron's probability wavefunction must unrealistically spread into infinity in y and z direction.
Because when the electron's probability wavefunction (= whose wave shape is related to de Broglie wavelength or electron's kinetic energy ) is confined in a limited area also in y or z direction, it causes the electron's kinetic energy due to finite de Broglie wavelength related to electron's momentum (= kinetic energy ).
↑ So in the unrealistic quantum mechanical probability wavefunction, each electron can Not move linearly (= in x direction, so wavefunction infinitely spreading in y,z directions ) or circularly (= in x,y direction, so wavefunction infinitely spreading in z direction ), which contradicts the fact or experiments where electrons can move linearly in confined area.
As a result, the quantum mechanics or its probability wavefunction is proven wrong.
(Fig.4') Quantum mechanics can Not avoid unreal negative kinetic energy.
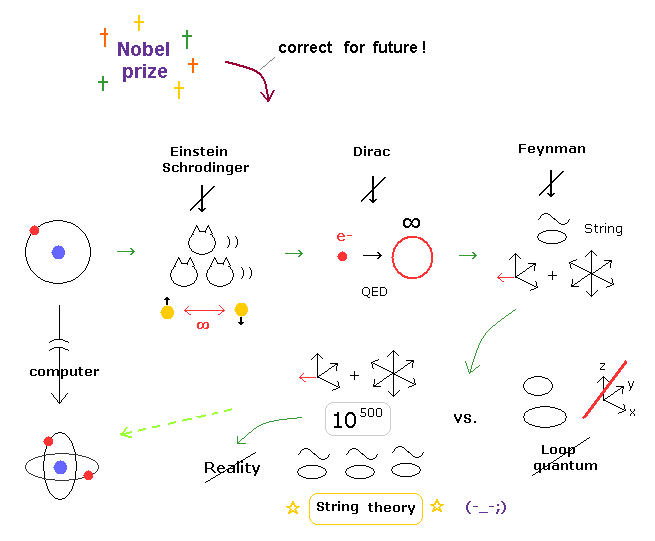
Quantum mechanical atomic wavefunctions must always include unreal negative kinetic energy ( this p.2-3 ), which fact disproves quantum mechanics.
As shown in the upper figure, it is impossible to eliminate the unreal negative kinetic energy probability area.
To eliminate the unreal negative kinetic energy electron's probability, the probability of electrons with zero kinetic energy must be zero (= probability wavefunction of ψ(r1) at r1 point in this must be zero ) so that electrons cannot go beyond the zero-kinetic energy region.
But according to quantum mechanical Schrodinger equation, the kinetic energy (= k(r1) ) expressed as second-order derivative of probability wavefunctions can never be zero at the point r1 of the zero-probability wavefunction.
↑ Because the denominator (= zero probability amplitude of wavefunction ψ(r1) = 0 ) of the Schrodinger equation's kinetic energy (= k(r1) ) must be zero, which prevents the zero kinetic energy (= kinetic energy k(r1) is not zero due to its zero denominator ) at the point of zero-probability wavefunction ( ψ(r1) = 0 ).
↑ When the numerator ψ''(r1) (= second-derivative of ψ(r) ) of kinetic energy (= k(r1) ) is zero, the denominator ψ(r1) is also zero (= for example, when the numerator ψ''(r) = x - r1, the denominator ψ(r) always becomes ( x-r1 )^n, n > 1 ), and the total kinetic energy (= k(r1) = ψ''(r1)/ψ(r1) ) becomes nonzero, or infinite.
As a result, the protability of an electron with zero kinetic energy must always be non-zero, followed by the unreal negative kinetic energy region (= if this negative kinetic energy area is artificially made to be suddenly zero, the wavefunction becomes discontinuous, and the kinetic energy on the edge becomes infinite or non-zero, so electrons cannot stop, which is self-contradictory ).
(Fig.5) Electron spin can be replaced by a real electron's orbit.
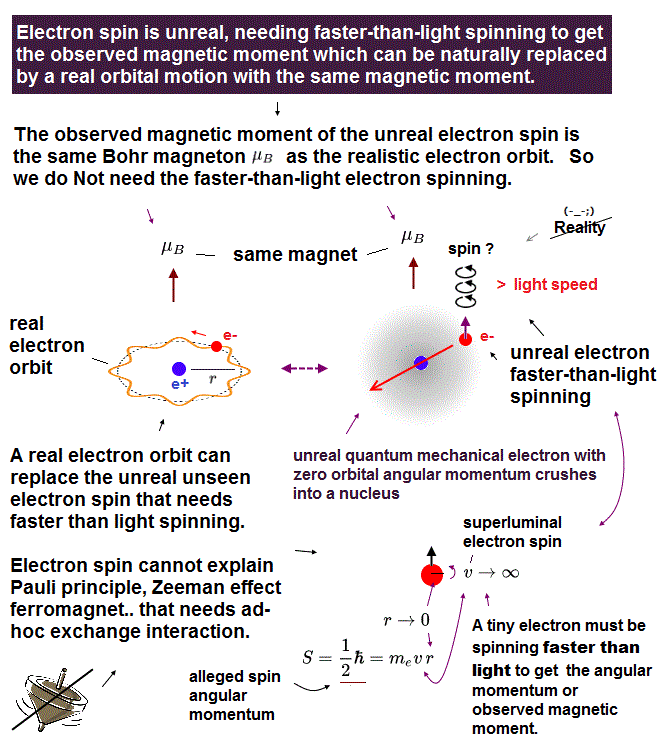
An electron spin is unreal, Not a real spinning, which contradicts the concept of spin angular momentum.
This (or this )-2nd-paragraph says -- Unreal electron spin
"electrons don't spin. They can't spin
If electrons actually spun.. their surfaces would be moving much faster than the speed of light"
A tiny electron, whose upper-limit size can be estimated from Coulomb scattering, must be spinning much faster than light (= disagreeing with Einstein relativity ) to generate the observed magnetic moment μB (= Bohr magneton ) or the alleged spin (unobservable) angular momentum of 1/2ℏ ( this-3rd-paragraph, this-p.2 ).
↑ angular momentum = mrv where electron's radius = r → 0 (= tiny electron ), v → ∞ (= unrealistic faster spinning speed v )
This-3rd-paragraph says -- Superluminal spin
"physicists soon determined that an electron would have to be spinning much faster than the speed of light (not allowed in this cosmos) to produce the effects they were seeing"
Even if we assume the tiny electron's radius is as big as the proton's radius (= 1 fm = 10-15 meter ), which is close to the old concept of classical electron radius (= bigger than the proton's radius or actual tiny electron, this-p.2-3rd-paragraph ), the spinning electron's surface speed must be about 100 times faster than the light speed ( this p.16, this p.4, this p.2-p.3, this p.3-last-paragraph, this p.2 ).
So quantum mechanics just vaguely and paradoxically says the (unrealistic) electron spin is just some intrinsic property irrelevant to actual spinning ( this-p.1-lower ).
Quantum mechanical Schrödinger equations are unreal, useless, unable to get analytical solutions except for a hydrogen atom whose energies agreed with the realistic Bohr atomic model.
The problem is the quantum mechanical unrealistic hydrogen model needs to have zero-orbital angular momentum (= electrons crash into nuclei ) that disagrees with experimentally-observed magnetic moment μB called Bohr magneton of a hydrogen atom (= quantum mechanical zero orbital angular momentum cannot generate the observed magnetic moment ).
So quantum mechanics illogically started to say that an electron has spin whose magnetic moment accidentally agrees with the Bohr magneton of the realistic electron's orbit, though the electron is Not actually spinning ( this-p.2, this-3rd-paragraph ).
↑ Spin experiments can measure only this magnetic moment (= Bohr magneton of Bohr's orbit ) that can be explained by the real electron's orbit, so the (unreal) electron spin is unnecessary (= fictional spin's angular momentum or g-factor cannot be measured ).
Quantum electrodynamics (= QED ) relying on unreal infinite virtual particles with imaginary mass just gives useless infinities which can Not predict anomalous magnetic moment, contrary to hypes.
Electron spin is unnecessary and unreal, because electron spin disagrees with experiments.
Electron spin-spin magnetic energy is too weak (= 10-4 eV, this-p.41, this-p.10-left ) to explain strong Pauli principle repulsion (= ~20 eV = large repulsive energy is needed to exclude 3rd-electron with the same spin of lithium from inner 1s to outer 2s orbitals ), ferromagnet, singlet-triplet energy splitting (= ~ 3eV, this-p.8-upper ).
A one-electron hydrogen atom is known to show the normal Zeeman effect without electron spin.
Alkali atoms are said to show anomalous Zeeman effect whose energy level splitting under magnetic field can be explained by electric interaction with inner electrons Not by the unreal electron spin.
Because the energy level doublet splitting of alkali atoms' D-lines called fine structure (= related to anomalous Zeeman effect ) is too large to explain by the (unreal) electron spin-orbit magnetic interaction.
↑ So anomalous Zeeman energy splitting of alkali atoms such as sodium is caused by electric interaction with inner electrons, Not by the (paradoxical) spin-orbit magnetic interaction.
The fine structure energy splitting (= between 2p1/2 and 2p3/2 energy states ) of hydrogen-like atoms and lithium can be naturally explained by the realistic Bohr-Sommerfeld model without spin that was copied wrongly by the unreal quantum mechanical Dirac hydrogen fine structure ( this-p.14, this-lower ).
And the ad-hoc quantum mechanical spin Lande g-factor disagrees with the actual anomalous Zeeman energy splitting or atomic magnetic moments.
Giant magnetoresistance used in hard disc representing today's (deadend) spintronics was unexpected discovery irrelevant to quantum mechanical spin prediction ( this-p.3-2nd~3rd-paragraphs ), which can be naturally explained by electron's orbits' collision instead of the unrealistic spin.
To explain the disagreement between the (unreal) electron spin's tiny magnetic energy and the experimentally-observed large energy such as Pauli principle, ferromagetic, singlet-triplet energy splitting, quantum mechanics needs to artificially create the unphysical exchange energy ( this-p.7, this-p.7-8, this-p.10 ) lacking exchange force ( this-p.9-upper, this-p.8-lower ).
↑ This quantum mechanical exchange energy lacking real force is unreal, based on the pseudo-kinetic energy change caused by a single electron unrealistically existing in different atoms simultaneously in unphysical antisymmetric wavefunctions that must be artificially chosen, so cannot predict Pauli principle energy nor ferromagnetism, either..
Useless quantum field theory or Dirac equation just shows the unphysical abstract anticommutation that can Not clarify real Pauli principle mechanisms.
Unlike the unrealistic superluminal electron spinning, the nucleus, which is much bigger and heavier than a tiny electron, can be actually spinning to generate the observed very tiny magnetic moment even without relying on the faster-than-light spinning.
So MRI and atomic clocks can be explained by the classical nuclear spinning that does Not need the paradoxical quantum mechanical (unreal) spin.
Quantum mechanics and quantum electrodynamics (= QED ) are unable to explain the nuclear or proton spin (= causing tiny hyperfine structure energy splitting ) or masses ( this-p.4-right-lower, this-p.1-abstract ).
Physicists had to artificially create ad-hoc quantum chromodynamics (= QCD ) based on unreal imaginary time ( this-p.13 ) and many free parameters that can Not predict nuclear spin (= hyperfine structure ).
(Fig.6) The unrealistic electron spinning faster than light needs two rotations (= 720o ) to return.
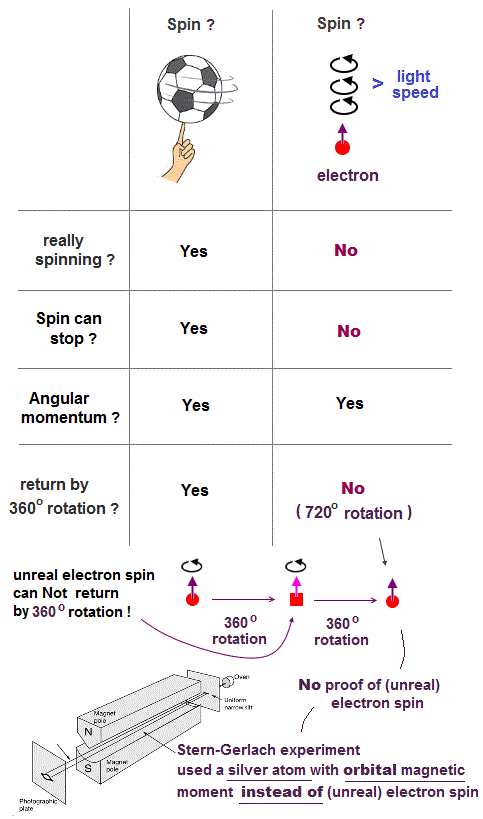
Unrealistic electron spin must be "spinning" much faster than light ( this p.3-lower, this p.4 ) to generate the designated angular momentum and magnetic field which accidentally agreed with Bohr magneton ( this p.2, this left ) given by Bohr model.
The famous Stern-Gerlach Experiment, which was supposed to measure electron spin's magnetic field, did Not measure an electron spin itself.
Stern-Gerlach experiment showed a "silver atom" has magnetic moment equal to Bohr magneton ( this p.5-1.17-lower, this p.16 ) which can be naturally explained by electron's orbital motion (= of real Bohr model ) instead of unreal spin.
↑ A realistic electron's orbital motion also split into two energy levels (= depending on whether electron's orbit is parallel or anti-parallel to the external magnetic field. The electron's orbit perpendicular to external magnetic field is unstable, as Bohr said in this p.14-1st-paragraph )
Electron spin is Not a real "spinning", because spinning must be unrealistically faster-than-light, and the spin cannot stop or slow down.
This quantum mechanical illogical claim that the spin should Not be treated as electron's spinning clearly contradicts another quantum mechanical claim that spin has "angular momentum", which must be generated by a "spinning" object.
More unreasonable thing is electron spin needs to rotate twice (= 720o ) instead of once to return to its original state. ← No physical mechanism can be given to such an uncanny spin which is unable to return to the original state just by 360o rotation.
The (spinning) electron really does Not return to its original state by the ordinary 360 degree (= or 2π ) rotation, instead, it needs 720 degree rotation (= two full rotations, 4π ) to return ?
Useless quantum mechanics refuses to offer any deeper physical mechanisms of this unreal electron spin ( this p.3-4 ).
Some experiments claimed that this physically-impossible property of electron spin which needs 720o (= instead of 360o ) rotation to return to the original state was confirmed by rotating (= precessing ) neutron spin interference.
But of course, they could Not see each neutron spin actually spinning (= because spin is Not an actual spin ). They just imagined the neutron rotated twice on the false assumption that each neutron has 1/2 ℏ spin angular momentum (= angular momentum itself cannot be directly measured, only magnetic field = Bohr magneton can be measured ).
If we assume each neutron's rotation has 1 ℏ angular momentum (= instead of 1/2 ℏ ) like real Bohr's orbit, this experimental result can be naturally interpreted as the one showing neutron normally returned to its original state by rotating once (= 360o ) instead of unrealistic 720o
Because precession speed is inversely proportional to angular momentum L as seen in gyroscope, so as a neutron's angular momentum increases from quantum mechanical 1/2 × ℏ to classical 1 × ℏ, precession velocity decreases from 720o to 360o rotation.
↑ These experiments of neutron precession and interference use the neutron's de Broglie wave interference ( this 7th-paragraph ) under applied external magnetic field ( this p.10, this p.6, this p.5 ).
The orbital motion based on de Broglie wave interference generates the quantized angular momentum = an integer times ℏ (= orbital angular momentum becomes 1 × ℏ instead of the unseen spin angular momentum's 1/2 × ℏ ) to avoid destructive interference of de Broglie wave by the orbital circumference equal to an integer times de Broglie wavelength (= by replacing the electron's mass with the neutron mass, you can get the same quantized angular momentum of 1 × ℏ ).
The precession speed (= inversely proportional to the spin or orbital angular momentum ) becomes two times lower (= slower = 360o precession or rotation ) when we consider the neutron's (orbital) angular momentum is 1 × ℏ than when we (falsely) use the neutron's 1/2 × ℏ spin angular momentum (= two times faster false 720o precession, this 10.5.4 ).
As a result, these types of experiments based on neutron's interference just proved all particles such as neutrons returned to their original states by the ordinary classical 360o rotation instead of fantasy quantum mechanical 720o rotation (= which unrealistic wrong interpretation is caused by the false assumption of the unreal unseen spin's 1/2 angular momentum causing two times faster false precession ).
A neutron is known to be a composite particle consisting of a proton and an electron, and the magnetic moment of electron spin is far larger than the magnetic moment of proton. So if an electron's spin is real, the magnetic moment of a neutron should be as large as an electron.
But an actual neutron's magnetic moment is as small as a proton (= an electron's orbital radius is as small as that of proton inside neutron, which causes negative weak magnetic moment which is almost the same as a proton's magnetic moment ), which means an electron's spin is unreal (= electron's orbital motion is real ).
In conclusion, electron spin with 1/2 angular momentum is physically impossible. Atomic powerful magnetic field is produced by a large electron's orbital motion instead of illusory electron's "spinning".
(Fig.8) Hydrogen shows "normal Zeeman effect" without spin.
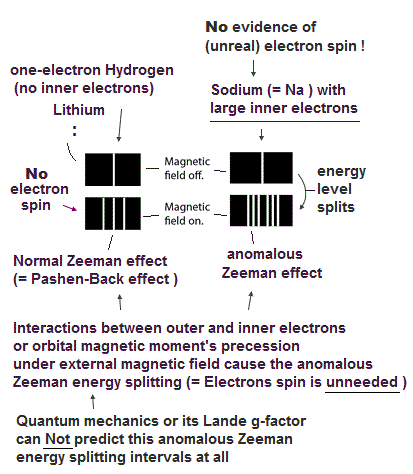
By measuring the energy or frequency (= wavelength ) of light emitted from or absorbed into atoms in spectral lines, we can know the atomic energy levels.
Under external magnetic field, the atomic energy levels are known to split into more energy levels due to different magnetic energies between the orbits in different directions and external magnetic field.
When there is No electron spin, each atomic energy level (with orbital angular momentum) under magnetic field is basically split into three , which is called normal Zeeman effect.
Various atoms such as hydrogen ( this p.3-left-3rd-paragraph ), even many-electron cadmium (= Cd, this p.4-1st-paragraph ), mercury ( this p.4-4th-paragraph ) are known to show this normal Zeeman effect, which clearly proves there is No electron spin.
Quantum mechanics unfoundedly says when very very weak magnetic field (= whose energy is less than tiny, tiny fine structure energy of 0.000045 eV ) can be applied to the hydrogen atom, it could split into more spectral lines (= anomalous Zeeman effect, this p.3-upper ) which may be a sign of (fictional) electron spin.
↑ But in hydrogen or lithium atoms, it is impossible to directly measure anomalous Zeeman effect energy splitting, so No evidence of electron spin.
Furthermore, if electron's orbit's precession is considered, the electron's orbit without spin can also explain more complicated spectral lines or anomalous Zeeman effect, so we do Not need electron spin after all.
It is impossible to precisely measure such a tiny, tiny energy splitting smaller than the fine structure or distinguish them from other thermal fluctuation, orbital precession or nuclear magnetic field effect in hydrogen or lithium atoms (= so textbooks' explanation of anomalous Zeeman effect uses only the sodium instead of the hydrogen ).
↑ The fact that anomalous Zeeman effect is seen only in large Alkali atoms such as sodium means the anomalous Zeeman effect is caused by electric interaction between inner electrons and a valence electron, Not by the (unreal) electron spin.
When applying the magnetic field larger than the tiny fine structure energy (= correctly, even when the applied magnetic field is slightly larger than much-weaker nuclear hyperfine energy ), all atoms are said to conveniently show normal Zeeman effect called Paschen-Back effect ( this p.14 ) disagreeing with anomalous Zeeman Lande g-factor. ← No evidence of electron spin.
↑ Hydrogen and lithium atoms are known to show only this normal Zeeman or Paschen-Back effect ( this-lower, this p.7-2nd-paragraph, You can Not find pictures of anomalous Zeeman effect splitting in hydrogen or lithium atoms in any textbooks or websites ), which means there is No electron spin.
(Fig.9) Anomalous Zeeman effect is Not by electron spin but by electric interaction with inner electrons.
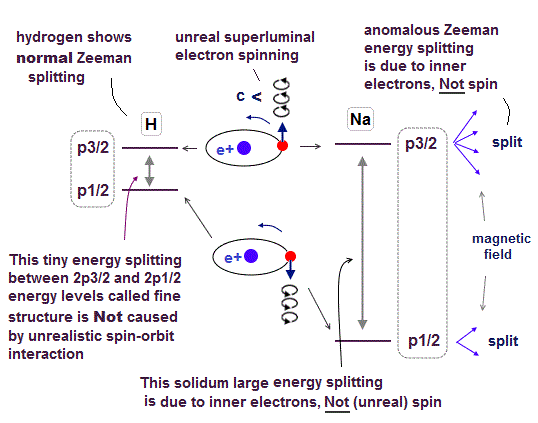
Sodium D-line = energy levels' transition from 3p3/2 or 3p1/2 excited states to 3s ground-state is known to split into multiple more energy levels under magnetic field, which is called anomalous Zeeman effect.
This anomalous Zeeman effect is said to show the sign of (fictional) electron spin, but actually, this anomalous Zeeman energy splitting is caused by (Coulomb) interaction between inner and outer electrons, Not by electron spin.
Actually, one-electron hydrogen atom without inner electrons shows normal Zeeman effect (= they call this Paschen-Back effect, which is substantially equal to normal Zeeman effect, which is seen also in lithium atom with a small number of inner electrons, this-lower ).
Sodium D-line or fine structure is said to be caused by relativistic spin-orbit (effective) magnetic interaction.
But this relativistic spin-orbit magnetic interaction is paradoxical and too weak to explain sodium or alkaline large D-line energy splitting.
For the (illusory) spin-orbit magnetic interaction to explain the sodium's large fine structure energy splitting between 3p1/2 and 3p3/2, the effective charge of Na+ (= singly-ionized sodium ) must be unrealistically bigger (= +3.54e ) than actual values of Na+ = +1.84e obtained from the experimental sodium's ionization energy (= electron spin or spin-orbit interaction disagrees with experimental results ).
As a result, the Alkali (= Na, K, Rb.. ) large fine structure energy splitting and anomalous Zeeman effect are Not caused by electron spin (or spin-orbit interaction ), but by electric interaction between inner electrons and a valence electron compatible with real Bohr-Sommerfeld model.
Quantum mechanical Schrodinger equations are wrong, unsolvable, unable to predict any multi-electron atoms such as sodium.
(Fig.10) For spin-orbit interaction to cause large sodium's fine structure energy splitting (= 0.0021eV ), the effective charge of singly-ionized sodium (= Na+ ) must be unrealistically bigger = +3.54e than actual Na+ charge of +1.84e.
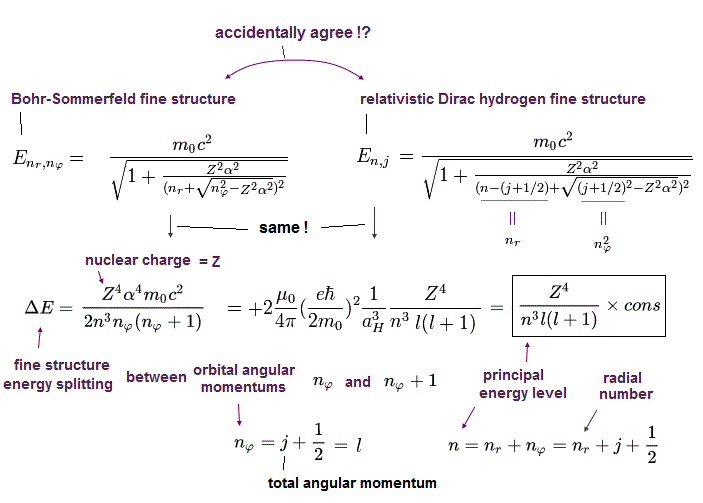
Quantum mechanics says anomalous Zeeman effect of sodium (= Na ) D-line (= fine structure energy splitting ) is caused by relativistic electron spin-orbit magnetic interaction (= relativity is paradoxical, so this spin-orbit interaction is unreal ).
But in fact, this sodium D-line energy splitting or fine structure is too large for the tiny relativistic spin-orbit magnetic interaction to explain.
Relativistic spin-orbit magnetic energy splitting or fine structure formula is proportional to Z4/n3 where Z is effective charge that must be close to 1 (= Z is the sum of nuclear positive charge and all inner electrons except for one outmost valence electron ), and "n" is principal quantum number related to energy level ( this p.9, this p.7, this-p.16, this-p.1.p.9-(1.37), this-11~12th-paragraphs ).
This-p.3(4)-p.4-TableI (or this-p.2~3 ) says -- Spin-orbit energy
"the energy of flipping the spin between two states
(spin up and spin down = quantum mechanical fine structure energy splitting ) can be expressed in Z4/n3... Z is the effective central
charge, n is the principal quantum number"
Hydrogen's fine structure energy splitting between 2p3/2 and 2p1/2 ( n = 2, Z = 1 ) is very tiny 0.000045 eV.
Sodium (= Na ) D-line or fine structure energy splitting between 3p3/2 and 3p1/2 ( n = 3, ) is very big 0.0021 eV ( this p.5-Table.I, this p.5-Table 1 ).
From these values, if Na D-line energy splitting is caused by tiny relativistic spin-orbit magnetic energy, the total charge of Na+ ion (= Na nuclear + all inner electrons, which must be close to 1 ) must be unrealistically big = +3.54 ( this p.3-upper, this-p.7 ), which disagrees with actual Na+ charge of +1.84 obtained from the experimental sodium's ionization energy.
(Fig.10') If Sodium (= Na ) D-line energy splitting between 3p3/2 and 3p1/2 is due to spin-orbit interaction, the charge of Na+ ion must be unrealistically large +3.54e instead of +e. ← Na D-line is due to Coulomb interaction instead of spin-orbit magnetic energy.
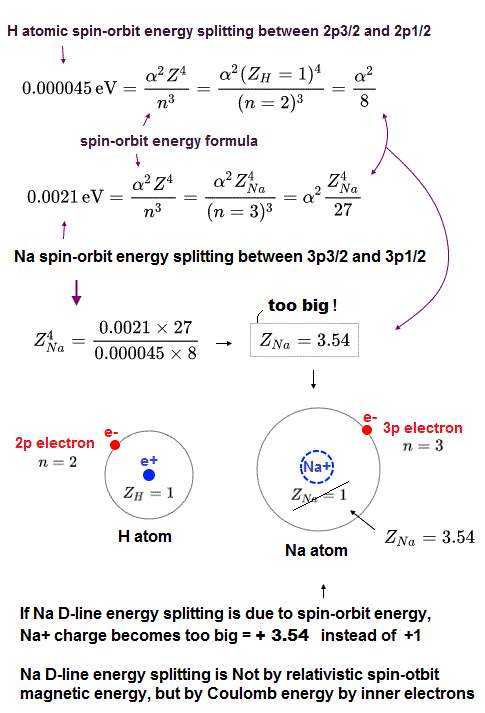
Disagreement in Na+ effective charges Z between one obtained from the (illusory) spin-orbit interaction (= Z = + 3.54 ) and the experimental sodium's ionization energy (= Z = 1.84 ) shows the sodium D-line energy splitting and anomalous Zeeman effect are irrelevant to electron spin, they are caused by Coulomb interaction between inner electrons and an outer valence electron.
We can know true effective central charge of this Na+ ion (= Na nucleus + all inner electrons except one outer electron ) using experimental ionization energy values.
Both hydrogen and sodium atoms have the similar structure with only one valence (= outer ) electron, so we can use the common ionization energy formula where the total energy is proportional to Z2/n2 where n is principal quantum number, Z is effective central charge ( this-p.3.p.13, this-lower ).
In hydrogen atom, the nucleus is +e, so Z = 1, and ionization energy is 13.6 eV ( energy level quantum number n= 1 ).
In sodium atom, the ionization energy of outer electron ( n = 3 ) is 5.14 eV.
Putting all these experimental values and quantum numbers into the energy formula (= ionization energy = Z2/n2 ), we can get the true effective central charge Na+ equal to +1.84 (= based on experimental ionization energy, this p.5 upper, this-problem 3 ), which is far smaller than the wrong Z = +3.54 (= obtained by assuming fictitious spin-orbit interaction causes very wide Na fine structure energy splitting ).
This huge discrepancy in effective central charges Z between experimental value and quantum mechanical spin theoretical value clearly proves that sodium D-lines = fine structure energy splitting is Not caused by fantasy electron spin, but by stronger Coulomb interaction between inner and outer electrons.
In all other alkaline and alkaline-earth atoms, their D-line and triplet energy splittings are too wide to explain by tiny spin-orbit and spin-spin magnetic interaction.
So the electron spin, which contradicts experimental results, does Not exist.
(Fig.11) Na+ real effective charge (= Na nucleus + all inner electrons ) is + 1.84e obtained from the experimental ionization energy of sodium.
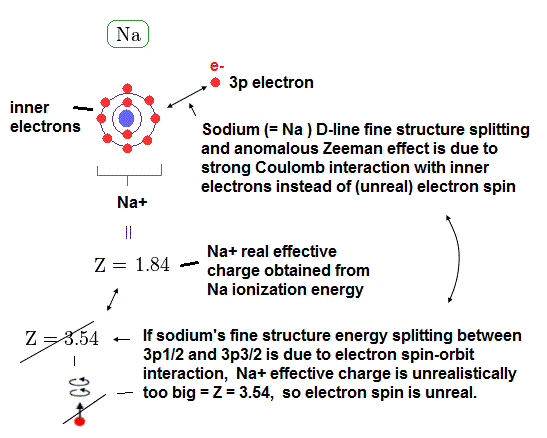
An outer electron, which causes the very wide fine structure energy splitting in sodium, can be considered to be orbiting around the effective central charge Z (= close to +1 ) of a singly-ionized sodium (= Na+ ) that combines a positive sodium nucleus and all other inner electrons except one outer electron.
A sodium's outer (= valence ) electron is moving around an singly-ionized sodium (= Na+ = effective central charge Z = 1 ).
Electrons are avoiding each other by Coulomb repulsion, so this effective central charge Z ( felt by an outer electron ) tends to be bigger than +1.
From the experimental ionization energy of the sodium, we can know the real effective charge of Na+ is +1.84e, which disagrees with +3.54e obtained from the (illusory) spin-orbit interaction in sodium's large fine structure energy splitting.
↑ This results show the Alkali (= Na, K, Rb.. ) large fine structure energy splitting and anomalous Zeeman effect are caused Not by the electron spin or spin-orbit interaction, but by the electric interaction between inner and outer electrons without spin, compatible with real Bohr-Sommerfeld model (= inner electrons of sodium makes its fine structure splitting between different orbits larger than the hydrogen ).
All other alkali and alkaline-earth atoms also show remarkable discrepancy between experimental values and prediction by quantum spin's theory (= spin-orbit or spin-spin magnetic interaction is too weak to cause wide energy splitting of alkali and alkaline-earth atoms ).
Hence, all quantum mechanics, spin and relativistic spin-orbit interaction turned out to be false, disagreeing with experiments.
(Fig.L-1) ↓Quantum mechanical spin or Lande g-factor relation is violated in anomalous Zeeman effect such as sodium D-line fine structure.
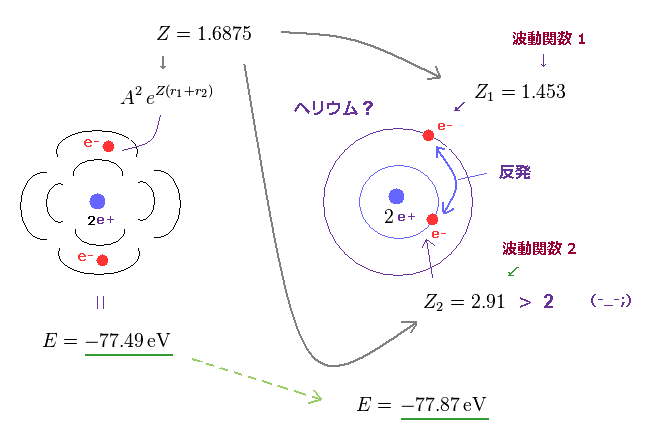
Anomalous Zeeman effect is said to obey Lande-g-factor (= gJ) or LS (= spin-orbit) coupling (empirical artificial) rule ( this p.11 ) consisting only of electron spin (= S ), orbit (= L ) and total angular momentum J = S + L ( this-p.6 ).
But actual anomalous Zeeman effects are much more complicated, and there are many cases disobeying Lande g-factor or LS coupling ( this p.4-last, this p.8-upper, this p.13, this p.140(or p.129)-1st-paragraph ).
This is why many atoms such as hydrogen, helium, potassium, rubidium whose exact Lande gj factors are unavailable ( this p.2-left-2nd-paragraph, this p.9-table.10, this p.32(p.28)-last~p.33 ) due to disagreement with Lande g-factor.
So physicists artificially changed the rules and fabricated ad-hoc J-J, J-K coupling rules (= changing the original LS coupling Lande g-factor rule ) to explain complicated inconsistent anomalous Zeeman energy splitting ( this p.22, this p.19, this p.3-4 ).
As a result, there is No evidence of electron spin based on artificial quantum mechanical rule or Lande g-factor.
(Fig.L-2) Interpretation of anomalous Zeeman energy E splitting under magnetic field B needs freely-chosen parameters A,B and the influence of irrelevant nuclear spins I,F, which cannot validate original Lande electron-spin g-factor gJ
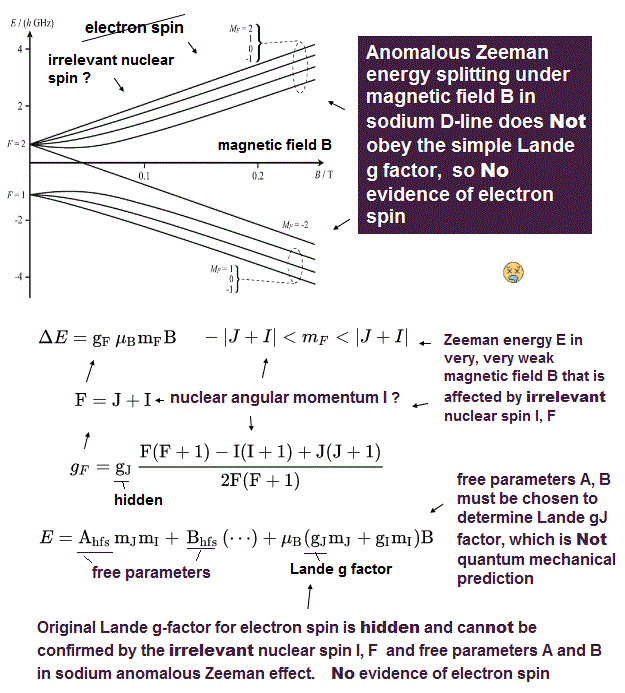
If Lande g factor (= gL or gJ ) based on (fictitious) spin-orbit coupling was right, D-line must split symmetrically with respect to magnetic field, but actually, they split unsymmetrically, more complicatedly ( this p.3-Fig.7-31, this p.38-39, this p.2-right-last ), which disagreed with Lange g factor formula.
Contrary to the simple textbooks' explanation, the measurement of Lande g factor (= gL ) based on (fictitious) electron spin (= S ) and orbital angular momentum (= L ) interaction must be done in extremely weak magnetic field B where even very small nuclear spin's motion (= hyperfine ) effect cannot be distinguished from the (fictitious) electron spin.
↑ The magnetic energy splitting in anomalous Zeeman effect (+ Alkali D-line or fine structure ) must consider the irrelevant nuclear angular momentum number (= hyperfine spin ) such as I (= nuclear spin angular number ) and F (= I + J, J = S + L ), which cannot distinguish or prove the existence of electron spin S ( this p.3~p.5-(23)(24),p.25-26, this p.2~p.4-(9)(10) ), which contradicts the fact that nuclear magnetic moment is far weaker than electron spin ( this-p.4~p.9 ).
↑ Physicists can artificially choose nuclear spin I numbers (= electron-nuclear mixed g factor gF instead of original Lande gL or gJ factor must be used, so it is Not quantum mechanical prediction of electron spin ), and knowing the extremely-weak magnetic field is very difficult ( this-p.13-(35)(36) ).
↑ Nuclear spin magnetic moment is negligibly smaller than the magnetic moments of electron spin and orbit, so intentionally mixing the irrelevant weaker nuclear spin (= I ) and the bigger electron spin's magnetic moment (= S or J ) for artificial Lande gF factor or F (= I + J ) by treating them equally contradicts reality.
↑ The original Lande-g factor gJ using only the bigger magnetic moments of electron spin and orbit disagrees with Zeeman effect, so they intentionally mix and treat the irrelevant negligibly-weaker nuclear spin's magnetic moment with far bigger eletron spin-orbit, as if nuclear and electron spins had magnetic moment of almost the same magnitude (= gF ) paradoxically.
↑ When applying magnetic field slightly larger than the nuclear spin magnetic moment, the anomalous Zeeman effect No longer obeys the simple linear Lande g-factor splitting (= instead, non-linear, complex splitting, this p.11 ), which energy splitting needs artificial adjustment of free parameters such as A and B ( this p.5-(25)(26),p.25-26, this p.4-lower, this p.3-5.p.7,p.28-39, this-p.2-right-last ).
As a result, there is No evidence of electron spin based on quantum mechanical Lande g factor and artificial Hund rule. ← Quantum mechanics lacking consistent rules cannot predict anything.
All atoms whose atomic numbers are less than 10 (= Neon ) also disobey Lande spin-orbit g factor, so No evidence of electron spin.
Zeeman energy splitting experiments of only two atoms of oxygen (= O ) and nitrogen (= N ) were conducted so far, and even these O and N atoms showed patterns different from the textbook anomalous Zeeman effects based on the simple Lande g-factor ( this p.2 ), and they had to artificially mix the influence of irrelevant Paschen-Back effect (= close to normal Zeeman effect ) using new artificial rules and equations different from Lande g-factor.
This paper of O and N atomic Zeeman effect ↓
p.1-left says "Only Zeeman effects for elements of atomic number below Ne 10 from which (Lande) g-values can be derived are.. for O II (= oxygen ) .. for N II (= nitrogen )" ← All atoms except the oxygen and nitrogen clearly disobey Lande g factor or the typical anomalous Zeeman effect.
p.9-left says "5P term does Not obey Lande interval rule."
p.16~22 used new artificial equations or rules completely different from the original Lande g factor relation in Zeeman effect of O and N atoms.
p.22-left-VIII says "Although the Paschen-Back perturbation in N are much less than those in O, yet they must be taken into account in determining (Lande) g-values from experimental data."
p.25-left-last says "despite the fact that the term intervals.. do Not conform with the Lande rations"
↑ As a result, there is No evidence of electron spin experimentally or theoretically.
(Fig.12) Spin-spin magnetic energy (= 0.0001 eV ) is too small !
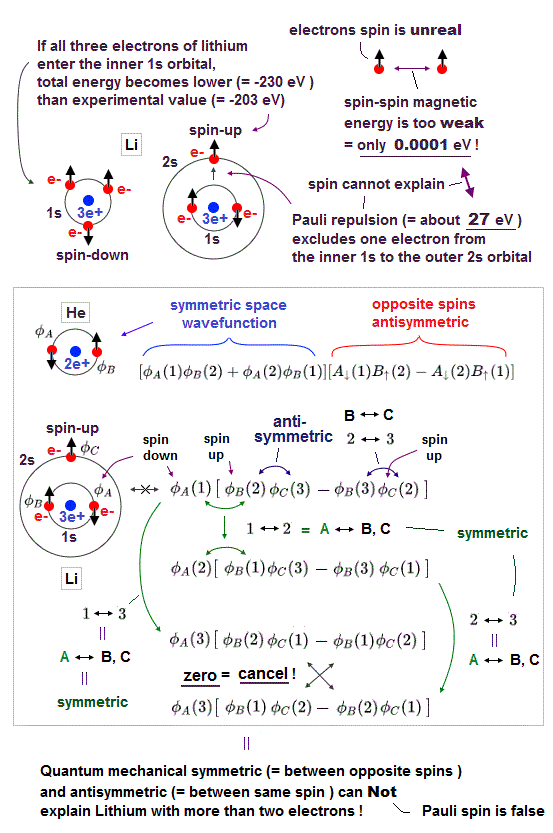
Quantum mechanical Pauli principle says two electrons with the same spin (= up-up spins or down-down spins ) can not occupy the same orbital.
So a helium atom (= He ) has two electrons with different up and down spins in the same 1s orbital.
And the third electron of lithium (= Li ) cannot enter the inner 1s orbital which always has two electrons with up and down spins and instead, it has to enter the outer 2s orbital due to Pauli exclusion repulsion (= though Pauli repulsion is Not a real repulsive force ).
But a electron spin is Not a real spinning.
And the electron spin-spin magnetic energy is known to be too weak to explain strong Pauli repulsive energy ( this p.5 ).
So the (unphysical) electron spin can Not explain Pauli exclusion principle.
Each electron is supposed to have the magnetic moment of Bohr magneton, which is accidentally the same as Bohr's realistic orbital magnetic moment ( this p.2 ).
The magnetic energy between two electron spins over the distance of 1Å (= 1 × 10-10 m ) is very small = only 0.0001 eV ( this p.17, this p.10-left-2nd-paragraph, this p.3, this p.3-p.4 ).
On the other hand, the Pauli repulsive energy required to exclude the 3rd electron of lithium from 1s to 2s orbital is as large as 20~30 eV ( this 2~3rd paragraphs ).
This p.1~2 shows the total energy of a fictitious lithium with all three electrons packed into the same inner 1s orbital is -8.4609 Eh (= -230.2 eV ), which is lower than true lithium energy (= -7.478 Eh = -203.4 eV ) with the 3rd electron in outer 2s orbital by 27 eV.
It means Pauli repulsive energy excluding the lithium 3rd electron from the inner 1s to the outer 2s orbital is 27 eV, which is far bigger than the electron spin-spin magnetic energy of only 0.0001 eV.
So the electron spin's magnetic energy can Not explain the strong Pauli exclusion repulsive energy.
(Fig.12') Quantum mechanical ad-hoc Pauli principle's antisymmetric wavefunction hampers each atom from having its shape.
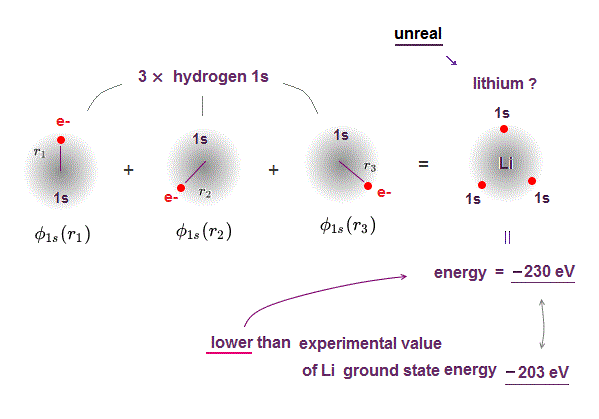
The wide discrepancy between the tiny spin magnetic energy and the strong Pauli repulsion made quantum mechanics artificially create an unphysical exchange energy ( this p.6-2.4, this p.7-8, this p.11 ) allegedly caused by unphysical antisymmetric wavefunction artificially chosen for unsolvable useless Schrodinger equations.
↑ Artificially choosing fake wavefunctions for unsolvable multi-electron Schrodinger equations means quantum mechanics cannot predict Pauli principle exchange energy, either.
This exchange energy is Not a real energy, because it lacks exchange force, so Pauli repulsion is Not considered to be a real force in paradoxical quantum mechanics ( this p.6, this p.10 ).
Because this exchange energy is caused Not by some potential or real force but by the fictitious electron's kinetic energy change.
↑ This quantum mechanical ad-hoc rule of the unphysical antisymmetric wavefunctions or exchange energy can Not clarify the detailed mechanism of Pauli principle, nor show any legitimate reason why the too-weak electron spin magnet has paradoxically-large power to exclude the 3rd-electron of lithium to the outer 2s electron.
Under the real repulsion, particles such as electrons should decelerate and their kinetic energy should decrease (= by feeling repulsion from other particles ).
But quantum mechanics paradoxically insists that electrons' kinetic energy should increase to cause Pauli repulsion in the unphysical antisymmetric wavefunctions.
This unphysical quantum mechanical Pauli exchange energy requires every electron to exist in all different atoms simultaneously (in artificial antisymmetric wavefunction ), which prohibits each atom from having real shapes as shown in the most-widely used one-pseudo-electron DFT model (= with artificially chosen exchange energy functional ), which unreal quantum mechanism model hampers development of science.
Relativistic quantum field theory based on unphysical Dirac equation just showing abstract anticommutation equation can Not explain the real mechanism of Pauli principle at all.
Electron's de Broglie wave is shown to interfere destructively (= pushing an electron out of the destructive interference area ) in two-slit and Davisson-Germer experiments.
↑ This realistic de Broglie wave destructive interference can be naturally used as the origin of real Pauli repulsive force without relying on paradoxical quantum mechanical exchange energy lacking real exchange force based on fictitious kinetic energy.
↑ Unphysical quantum mechanical Pauli repulsion also uses destructive interference of de Broglie wave, but in the wrong opposite way based on increased kinetic energy denying real Pauli repulsive force or force carriers ( this-p.9-upper, this-p.5-upper ).
This-(4) says -- Unreal exchange force
"The exchange interaction is sometimes called the exchange energy or exchange force. However, it is
Not a true energy or force." ← Quantum mechanical Paui principle based on fake exchange force is unreal.
(Fig.13) Electron spin is unreal and too weak to explain ferromagnet.
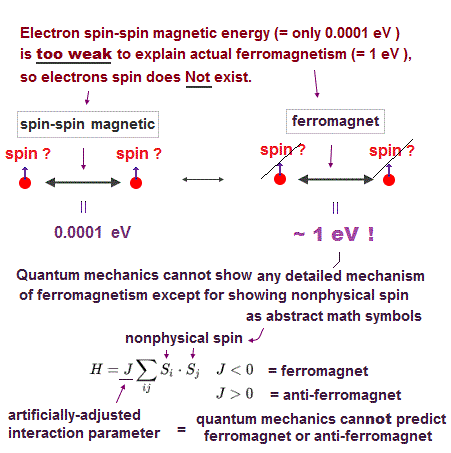
Ferromagnetism is said to be caused by electron spins aligned parallel to each other.
But in fact, ferromagnetism (or antiferromagnet, paramagnet .. ) has nothing to do with electron spin, because the magnetic interaction (= magnetic dipole-dipole energy ) between electron spins is too weak to keep ferromagnetism stable even at room temperature ( this p.7(or p.6), this p.8(or p.7) ).
This 3rd paragraph says -- Unreal exchange energy
"Such an interaction (= spin magnetic dipole interaction ) is, in general, much too small to produce ferromagnetism. Instead, the predominant interaction is known as the (unphysical) exchange interaction ( this p.7-8, this p.11(or p.3 )-lower )."
↑ This quantum mechanical ad-hoc unphysical exchange energy, which did not exist in the original spin magnetic definition, lacks reality, because they paradoxically claim quantum exchange energy may exist, but there is No exchange force ( this p.8-last-paragraph, this p.5-first-paragraph, this-middle Exchange energy ). ← nonsense.
Ferromagnetism of iron (= Fe ) is known to keep stable even at high temperature = the critical temperature 1043 K.
But if electron spin-spin magnetic interaction is the origin of keeping ferromagnetism, the iron easily loses its ferromagnetic property even at extremely low temperature.
↑ Electron spin-spin magnetic (dipole-dipole) interaction energy = 0.3 K is far smaller than actual strong interaction energy required to make ferromagnetic atoms (= direction ) stable = 1043 K ( this p.8 (or p.7)- dipole-dipole interaction ).
So the electron spin (= magnetic moment ) is Not the origin of keeping ferromagnetism stable, which fact contradicts the original definition of electron spins which was introduced as ones causing tiny magnetic field ( this p.9-p.10, this p.1-upper ). ← The existence of electron spin (= magnet ) has No evidence due to experimental disagreement between weak spin magnetic moment and strong Pauli principle, ferromagnetism which were Not caused by (fictional) electron spin.
Electron orbital motion (= realistically causing magnetic field of ferromagnet ) interacting and meshing (= synchronizing ) with other neighboring electron's orbital motions through Coulomb electric force can keep ferromagnetism stable even at high temperature ( this p.11 ).
↑ The electron's orbital motion covers larger space than the point-like electron's spin, so the electron's orbit-orbit interaction covering larger space naturally involves strong Coulomb electric interaction between electrons to let two neighboring electron's orbits interact and synchronize with each other stably.
Quantum mechanical fictional spin model expressed just as nonphysical math symbols lacking real spin or particle picture can Not predict any (illusory) spin-related phenomena relying on useless Schrodinger equation, fictional quasiparticle model and one-pseudo-electron DFT model.
See this
(Fig.16) Double-slit experiments proved electron's wave interference obeying de Broglie wavelength.
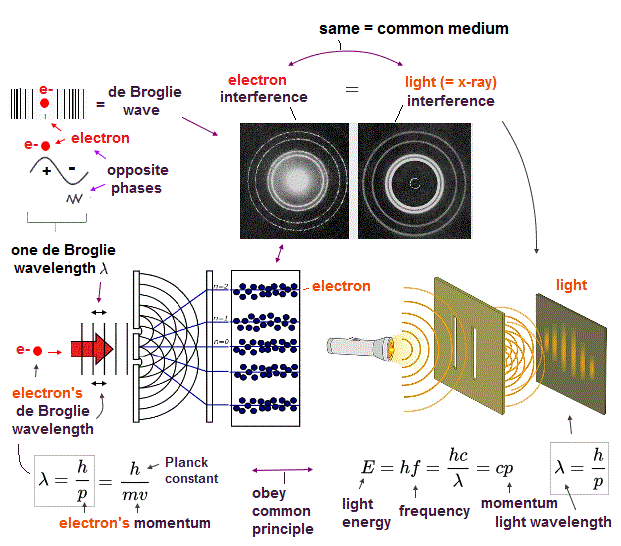
Many experiments confirmed that an electron has wave nature where an electron can interfere with itself obeying de Broglie relation wavelength in the same way as ordinary light wave.
Quantum mechanics claims even a single electron can interfere with itself, but if there is only a single electron in "completely empty space", it's impossible to cause interference fringe pattern, because an electron cannot voluntarily push or pull itself to change its own moving direction to cause interference fringe patterns on the screen.
So there must be some "external things or medium" exerting force on an electron by pushing or pulling an electron to cause fringes responding to destructive or constructive interference of de Broglie wave occurring around an electron.
Actually, Bohr model could successfully obtain atomic energy levels which just agreed with experimental results using de Broglie wave theory.
Later, quantum mechanical Schrodinger equation also used de Broglie wave theory and got exactly the same results as Bohr model.
And the realistic electron's de Broglie wave can naturally explain Aharonov-Bohm effect, magnetic flux quantum and quantum Hall effect even without relying on unreal fractional-charge quasiparticles of quantum mechanics.
(Fig.17) A single electron splits into parallel worlds !?
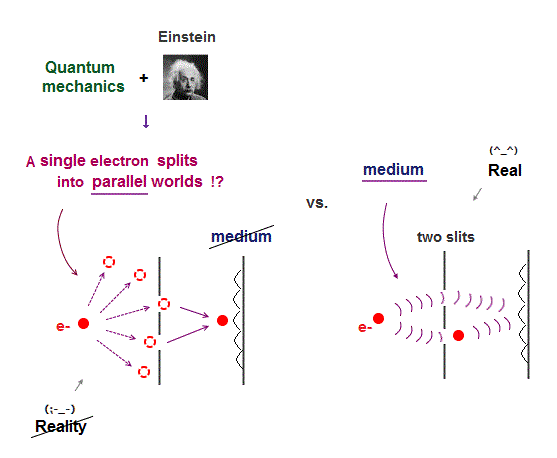
A single electron is obviously the smallest elementary particle with unbreakable mass and charge, which can Not be divided into multiple smaller charges also in two-slit experiments.
Each single electron is known to interfere with itself in two-slit experiment. ← Each single electron cannot be divided, then, how can the unphysical quantum mechanics explain this two-slit interference of a single electron ?
According to the ridiculous quantum mechanical logic, even a single indivisible electron particle ( or an imaginary photon ) must pass through two different slits or paths simultaneously. ← Impossible !
Of course, it's impossible for a single indivisible particle such as an electron to pass through two different slits at once to interfere with itself even in the ridiculous quantum mechanics.
Actually, each single electron is detected as a single electron at only one location one by one after passing through two slits and interfering.
So physicists have been unable to give realistic explanation of how a single particle can go through two slits without actually splitting, as an unsolvable mystery (= forever ), giving up realistic mechanisms ( this last-paragraph ).
Finally, the illogical quantum mechanics came to make an unrealistic claim that even a single unbreakable particle such as an electron must exist everywhere in different places simultaneously (= called superposition ) as if the single particle split into different fantasy parallel worlds or multiverse, creating its body-doubles.
So according to such an unscientific quantum mechanics, each indivisible electron particle could unrealistically split into fantasy different parallel universes or multiverse, pass through two slits at once, interfere with itself, and be detected as the original single electron in double-slit interference experiments.
Any other interpretation of quantum mechanics are Not different from the current mainstream (fantasy) many- or parallel-world theory where physicists basically avoid delving into the unrealistic quantum mechanical mechanism, instead, just reluctantly saying "Nobody understands (weird) quantum mechanics, so just shut up and calculate !" ← It's Not science !
For each single electron to cause the observed interference fringe, the single electron must be pushed out of destructive interference area by some external things, because a single electron itself cannot push or pull itself to dislocate itself according to the law of action and reaction (= two opposite forces of pushing and pulling cancel each other inside a single electron ).
So the only realistic explanation is that a single electron (or a photon = just classical light wave ) causes the real de Broglie wave (= which interference was experimentally confirmed as real ) in the external medium, and this de Broglie wave interference affects and dislocates the electron's position, resultantly showing the constructive and destructive interference fringes.
↑ This realistic picture of separating a particle and wave can naturally explain the actually-observed two-slit interference of a single electron without relying on fantasy quantum mechanical parallel worlds.
All experiments such as light wave interference, refraction, and diffraction of an electron clearly show the existence of real medium in space.
(Fig.18) Schrodinger's orbital is n × de Broglie wavelength.
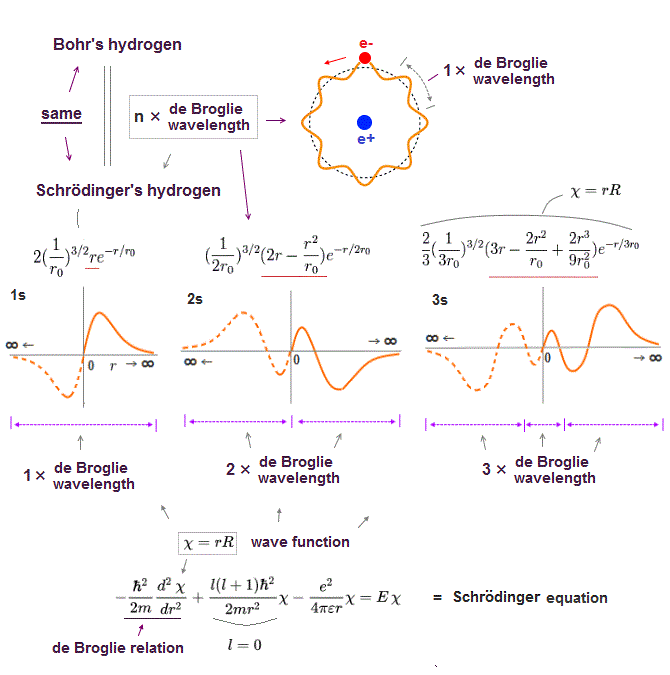
Bohr atomic model succeeded in explaining energy levels of all hydrogen-like atoms, and Schrödinger equation of quantum mechanics also agreed with results of Bohr model ( this p.13-lower, this p.7-lower ). Why ?
Bohr model uses the assumption that electron' orbital length is just an integral multiple of de Broglie wavelength to avoid destructive interferene, which was experimentally confirmed.
Schrödinger equation also uses de Broglie wave theory for obtaining electron's momentum and kinetic energy.
Furthermore, in fact, the electron's orbital of Schrödinger wave function is also an integer multiple of de Broglie wavelength ( this last ), which is the reason why both Bohr model and Schrödinger equation give exactly the same results and use the same Bohr radius concept in hydrogen.
We can visualize any Schrödinger's orbitals just equal to an integer times de Broglie wavelength like Bohr's atomic model.
Fig.18 is hydrogen's 1s, 2s and 3s wave functions.
If we use the solution χ = rR ( R is radial wavefunction, r is the distance between an electron and nucleus ), Schrödinger equation just becomes the simple second derivative form where Schrödinger's "radial wavefunction" exactly means "de Broglie wave" like Bohr model ( this p.3 ).
In this radial wave function (= rR ), 1s, 2s and 3s orbital are just integers = 1, 2, and 3 times de Broglie wavelength.
This is a hidden trick of the only solvable Schrödinger's solution = hydrogen atom, which results just agree with Bohr's hydrogen.
But only Schrödinger wave functions include unphysical orbitals, so false.
Because Schrödinger equation always has to include unrealistic zero orbital angular momentum, where an electron crashes into a nucleus, moving in a linear orbit.
In the linear orbit where an electron is moving back and forth on the same one-dimensional path, the electron's de Broglie wave interferes with itself destructively, hence, Schrödinger's electron's motion becomes unstable and chaotic, while Bohr's atomic electron's motion is stable.
(Fig.19) Quantum mechanical wavefunction is unreal.
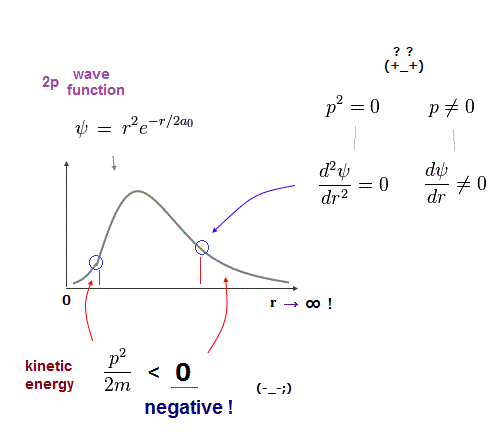
Quantum mechanics is false in hydrogen's wave function.
Because the square of momentum p of Schrödinger's electron is Not equal to p2, so, the equation of p2 = p2 is Not satisfied in quantum mechanics ? ← Why does such an irrational thing happen ?
Schrodinger equation adopted de Broglie relation as "derivative" form.
The first derivative of momentum operator acting on wavefunction gets the momentum p, and the second derivative of wavefunction gets the square p2.
Of course, when momentum p is zero, its square p2 must be zero, too.
But only when a wavefunction has basic " cos" or "sin" form, it holds true.
The point is quantum mechanical wave functions distort original de Broglie relation. Figure above is hydrogen 2p radial wavefunction ( this, this last ).
"2p" wavefunction has unreal negative kinetic energy on both sides.
On these boundaries (= two positions where electron's kinetic energy is zero ), the second derivative is zero ( p2 = 0 ), but first derivative (= p ) is not zero (= the slope of wave function is not zero, which means the momentum p is not zero ) ! This is ridiculous.
It's quite natural that when p is zero, its square p2 is zero, too !
So quantum mechanics distorts original de Broglie relation with wrong math.
(Fig.20) No solution → just "choose" fake solution ! = useless
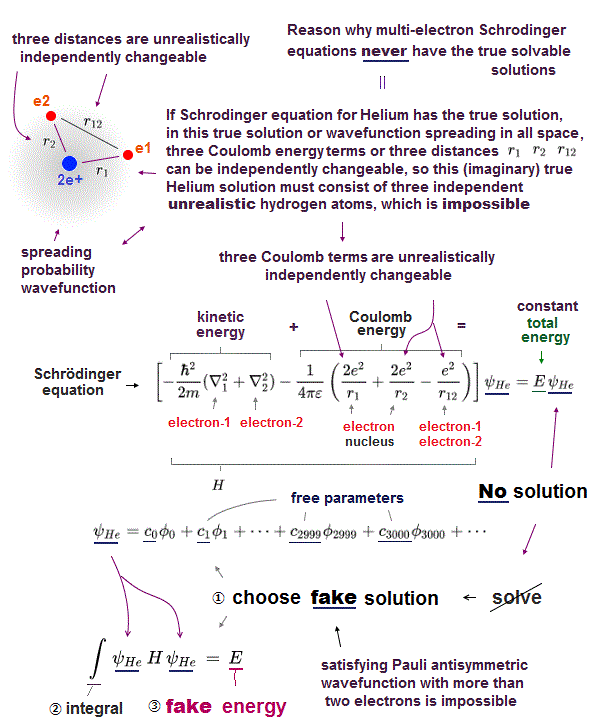
In Schrödinger equation of quantum mechanics, the sum of an electron's kinetic energy and Coulomb potential energy equals total energy E which total energy must be conserved and constant.
Under this total energy conservation (= E is a constant value ) law, Schrödinger equation can be solved only in one-electron hydrogen atom which results happened to equal realistic Bohr model.
In multi-electron atoms or molecules, Schrödinger equation can never be solved. = There are No exact solutions or wavefunctions of any multi-electron atoms and hydrogen molecule ion in quantum mechanics ( this 9th-paragraph, this p.4-1st-paragraph ).
So quantum mechanics gave up solving Schrödinger equation, and it just chooses and guesses fake solutions (= wavefunction ) called trial function or basis set, and integrates chosen fake solution (= fake chosen wavefunction ) with Schrödinger equation to obtain fake total energy ( this p.6-second-paragraph ).
There is 100% freedom in selecting the form of trial wavefunction or basis sets (= fake solution, this p.22-5.1 ). ← Even these fake chosen wavefunction solutions as the approximate methods for unsolvable Schrödinger equation are too complicated. time-consuming and impractical ( this p.14, this p.11 ), which make quantum mechanics useless in all applied science.
There is No limit to the number of parameters of trial wavefunction.
Variational methods normally try to find the (fake) trial function giving the lowest (= ground-state ) energy, but there are infinite choices of trial wavefunctions, so it's impossible to know the lowest energy based on this dubious variational method.
↑ You can freely choose any arbitrary fake solution and freely-adjustable parameters giving any arbitrary fake energies, which quantum mechanical methods cannot predict any true energies (= called variational methods, which are mainly used in almost-all quantum mechanical approximations with unsolvable multi-electron Schrödinger equations ), hence it's a kind of "art", Not science ( this p.17, this p.7 ).
When textbooks (often misleadingly) say contradictory things such as "solve approximate (unsolvable) Schrödinger equations or Hartree-Fock equations", physicists just artificially choose fake approximate wavefunctions (= called basis set solutions ) and manipulate their coefficients or parameters to get the lowest (fake) energies within chosen limited fake solutions called "variational methods ( this p.4, this p.8, this p.11 )" without actually solving them.
There is No way to know the exact atomic wavefunctions or solutions (= due to unsolvable multi-electron Schrödinger equations ) until physicists compare fake energies calculated from artificially-chosen fake solutions with the experimental energy values ( this p.4-5th-paragraph, this 2nd-last-paragraph, this p.1-last, this 3~5th paragraphs, this p.5-last-paragraph, this 15th-paragraph ).
↑ So it's far better to use the experimentally-obtained atomic energy values (and atomic shapes ) from the beginning without wasting time in artificially choosing and calculating fake energies using unsolvable multi-electron Schrödinger equations which can Not predict any atomic or molecular energies, hence quantum mechanical methods are meaningless ( this p.6-1st-paragraph ).
Textbooks often say impractical things; if you take infinite time to pick up some fake solution consisting of infinite freely-adjustable parameters and infinite terms, you may find true solution ( this 12th paragraph ). ← But this is Not true. No atomic true solutions can be found in quantum mechanics.
In larger atoms (such as Lithium ) and molecules with more than two electrons, the quantum mechanical Pauli antisymmetric wavefunctions become invalid and wrong.
No Schrödinger equations for multi-electron atoms such as helium or molecules can be solved, so the useless quantum mechanics has to artificially choose fictitious trial wavefunctions with an arbitrary number of freely-adjustable parameters as (fake) approximate solutions using the variational method.
The reason why these artificially-chosen fake approximate wavefunctions give total energies close to experimental atomic energies is they can just freely choose arbitrary convenient fake approximate wavefunctions out of infinite choices, and those chosen (fake) wavefunctions often consist of (irrelevant) one-electron hydrogen atomic wavefunctions ( this p.2, this p.6-upper, this-8.2.12 ). ← Remember that quantum mechanical Schrödinger's hydrogen atomic energy levels completely agreed with Bohr's atomic model
↑ Of course, one-electron hydrogen atomic wavefunctions (= ground-state and excited states ) are Not true solutions of multi-electron Schrödinger equations, and we prove that No multi-electron Schrödinger equations can have exact solutions (= conserving the total energy E in any electrons' positions is impossible in three-body quantum mechanical helium atom, so quantum mechanic is wrong. We have to compute two realistically-moving electrons' behaviors by directly calculating Coulomb forces between two electrons, using computers and realistic helium atomic model to conserve the total energy in any electrons' positions ).
↑ One-electron hydrogen atomic energies of the ground and excited states given by quantum mechanical Schrödinger equations completely agree with the realistic Bohr's successful atomic model.
Because both the successful Bohr's atomic model and the (unrealistic) quantum mechanical Schrödinger equation (= in the only solvable one-electron hydrogen atom ) use the same principle where the total energy is equal to the sum of Coulomb electric energy and electron's kinetic energy expressed as de Broglie wave ( this p.4-5, this p.8, this p.23-29 ).
So the fact that the unsolvable multi-electron Schrödinger equations could seemingly give the atomic energies close to experimental values using the (irrelevant) one-electron hydrogen atomic wavefunctions as (fake) approximate wavefunctions means that all atomic electrons' behaviors are governed by the common principle of Coulomb electric force and de Broglie waves (= both of which are used by realistic Bohr's atom which proved to be right ), and it does Not mean the (fantasy) quantum mechanics is close to reality.
(F.21) One electron exists in both H-atoms a and b at once ?
Quantum mechanical wavefunction always spreads symmetrically as electron cloud, so it cannot give strong Coulomb force for froming covalent bonds.
So quantum mechanics has to rely on unphysical exchange energy by forcing each electron to exist in any different atoms simultaneously.
(Fig.22) ↓ Each electron exists in all atoms unrealisically. → unphysical Pauli principle exchange energy integral.
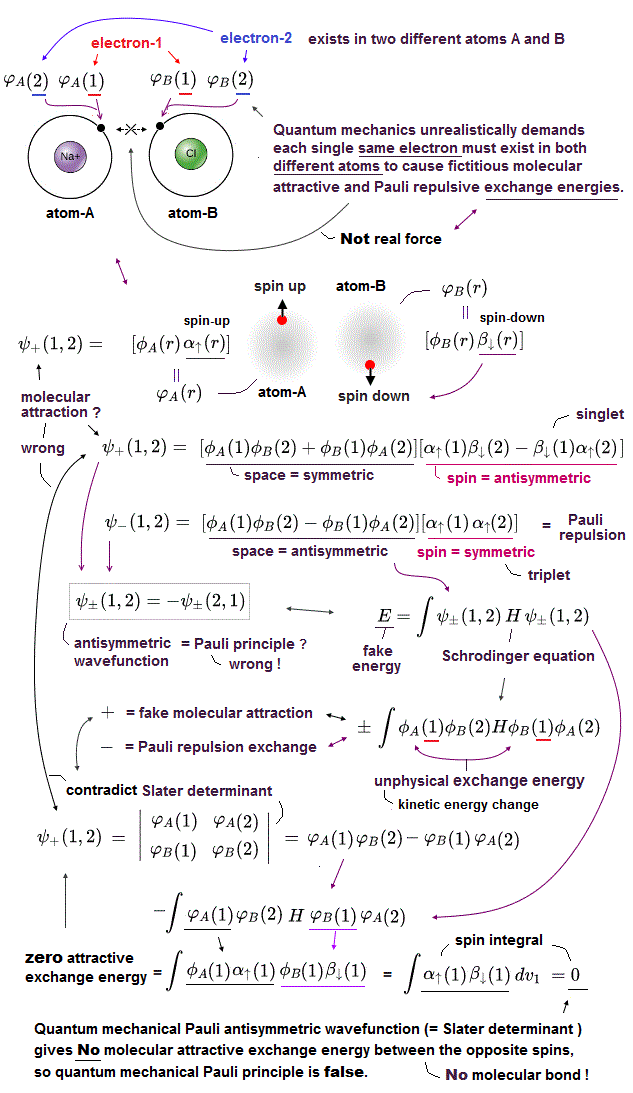
Pauli exclusion principle is known to generate mysterious powerful repulsive force enough to resist Coulomb force and exclude the 3rd electron of lithium to outer orbit, involving electron spin.
In fact, unphysical quantum mechanics cannot describe this Pauli exclusion principle or repulsive energy (= expressed as unphysical exchange energy ) using any real things or forces ( this-middle Exchange energy ).
So Pauli exclusion principle by quantum mechanics is based on wrong abstract nonphysical physics using fictitious concepts such as "exchange energy" and "antisymmetric wavefunction", which have nothing to do with real world.
Irrational quantum mechanical rule ( causing unphysical exchange energy ) forces all electrons to be indistinguishable, existing in all different atoms simultaneously ( this p.2, this p.3, this p.11 ) by making quantum mechanical wavefunctions take an artificial, unphysical form called "antisymmetric wavefunction" ( this 5th-paragraph, this p.18-(12)(13), this p.4-second-paragraph, this p.9 or p.6, this p.13-14 ).
In antisymmetric wavefunction, when we exchange any two electrons, the sign of the entire wave function is supposed to change ( this p.3 ). ← No more detailed mechanism of Pauli principle (except for forbidding two electrons in the same orbital or spin ) is given by quantum mechanics !
As shown in Fig.22 middle, the wavefunction is divided into two parts; electron's spatial part (= Schrödinger equation ) and spin part ( this-8.6.6-8.6.11, this p.16-18, this p.1-3, this p.6, this p.2 ).
In two-electron hydrogen (= H2 ) molecule or helium atom, the spin part is antisymmetric (= one electrons spin is up, the other spin is down, which is called "singlet" ), so their Schrödinger equation's spatial part takes "symmetric" form where a illegitimately-lowered electron's kinetic energy expressed as exchange energy is used as fake molecular bond energy in quantum mechanics ( this p.3-lower, this p.27, this p.3-last-paragraph ).
In antisymmetric wavefunction (= Pauli principle ) between the same spin-up-up or spin-down-down, the sign of this illegitimately-lowered electron's kinetic energy is flipped, which means the electron's "kinetic energy is illegitimately increased" in unphysical quantum mechanical Pauli principle or antibond ( this p.13 2nd-paragraph ).
So the origin of quantum mechanical Pauli exclusion force is nonsense = illegitimately-increased kinetic energy (= without using normal Coulomb repulsion or other forces, instead, using only unphysical "exchange" ) violating energy conservation law, so this unphysical Pauli repulsion can neither be admitted as real force nor given any realistic physical interpretation ( this p.6 ).
↑ Quantum mechanical Pauli repulsion based on the unphysical exchange energy or the increased kinetic energy ( this-p.9-10 ) contradicts reality, because the real (Pauli) repulsion should decrease the electron's kinetic energy when two repulsive electrons approach each other.
And this ad-hoc unphysical quantum mechanical exchange energy cannot give real force or force carrier to Pauli exclusion repulsion, while realistic atomic model with electrons and de Broglie waves can treat Pauli repulsive force as real force generated by real force carrier.
This unphysical Pauli exchange energy demanding every single electron exist in all different atoms makes a quantum mechanical atom borderless, shapeless, unrealistic, which needs artificial pseudo-potentials (= based on unphysical kinetic energy, Not giving each atomic shape) of one-pseudo-electron DFT, and extremely-time-consuming molecular dynamics, which hampers using atoms as real tools.
(Fig.23) Unreal indistinguishable electron → One pseudo-electron DFT approximation in quantum mechanics.
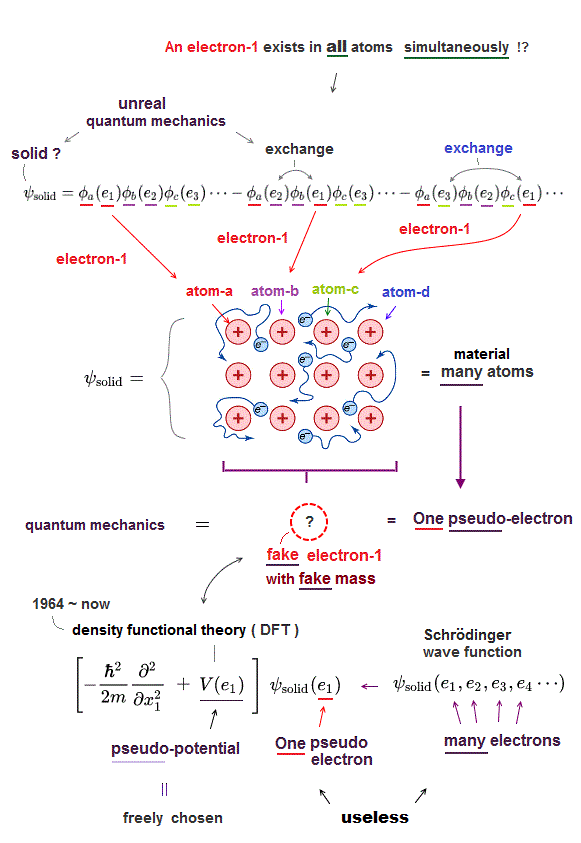
See DFT is wrong
(Fig.25) A single photon of radio wave with 1000 meter wavelength is unrealistically as big as 1000 meter ? ← this photon picture is impossible.
Quantum mechanics ridiculously insists that light can be a particle called a photon and wave at the same time, though this is physically impossible, a particle and wave are completely different things.
Many phenomena such as light interference, diffraction, refraction, and the constant light speed in the same medium (= like sound wave ) clearly show the light is electromagnetic wave, Not a photon particle.
If light was really a photon particle, physicists could have already given the concrete shape and size of a single photon particle, but still Nobody knows what size and shape a single photon is ( this p.1-right ).
For example, if the size of the radio wave photon with more than 1000 meter wavelength is as big as 1000 meter, such a big (illusory) photon can be easily seen and touched, but No such big photons were detected.
Actually, all textbooks can show a photon picture as wave, Not a particle.
Quantum mechanics ridiculously says that a single indivisible photon can interfere with itself by the single photon magically passing though multiple slits simultaneously using (fictional) quantum parallel worlds or superposition (= a dead-and-alive cat state ).
This p.2-2nd-last-paragraph says
"the (single) photon passes through both slits simultaneously and
when the single photon emerges through both slits, it interferes with itself (= impossible )"
↑ Even if a single "indivisible" photon can unrealistically split into two ghost photons in different parallel worlds, the (rigid) photon particles just clash into each other without causing interference pattern, which disagrees with experimental observation.
As Lamb said, there is No such thing as a photon particle ( this p.18 ).
Physicists try to misinterpret electrons excited by incident (classical weak) light wave in the photodetector as a sign of the fictitious photon.
↑ When the intensity of the weak light surpasses some (adjustable) detection threshold of causing electronic current in the photodetector, this excited electrons are treated as a (fictional) photon (= an illusory photon itself is unobservable ).
↑ Light with high frequency but with No intensity can Not eject electrons in photodetectors.
Light needs to have some minimum intensity to surpass the detection threshold of photodetectors.
This illusory photon particle, which is just light wave whose power surpassing the photodetector's detection threshold, causes unscientific quantum mechanical phenomena such as a single photon interference, superluminal entanglement, delayed choice quantum eraser, all of which can be naturally explained by classical light wave.
Relativistic QED says the vacuum is filled with unphysical virtual photons with imaginary mass, which paradoxically disobey Einstein relativistic mass-energy relation (= called off-mass-shell ), to mediate electromagnetic force ( this-p.3, this-p.16 ).
The current mainstream quantum field theory and quantum electrodynamics (= QED ) can only show a photon particle as nonphysical math symbols without concrete shapes nor sizes ( this p.4, this p.6-left ).
As a result, there is No such thing as a photon particle and imaginary mass.
All observed phenomena show Light is just wave traveling through the medium.
The unreal photon particle originates from paradoxical Einstein relativity rejecting the real medium.
(Fig.26) Light wave interacts with electron's de Broglie wave in real photoelectric effect, which can explain the light-frequency-dependent energy.
In fact, photoelectric effect (= electrons absorbing light energy are ejected from atoms ) and Compton scattering can be perfectly explained by classical light wave, Not by a (contradictory) quantum photon particle.
Textbooks often say the wrong explanation where the photoelectric effect showing the light energy is proportional to light frequency f indicates a photon particle, and if light is wave, it takes too much time for a tiny electron to absorb the light wave spreading over large area to cause photoelectric effect.
But first of all, the light frequency f is equal to c/λ where λ is light wavelength, which means light wave is related to photoelectric effect.
Furthermore, if a quantum mechanical photon is related to the photoelectric effect, an electron can absorb and emit only an unreal virtual photon with imaginary mass when the total energy and momentum are conserved ( this p.15, this p.10, this p.18, this p.3-5 ).
This means the quantum mechanical photoelectric effect dealing only with paradoxical virtual photons is unreal and wrong.
We can avoid these unrealistic quantum mechanical virtual photons, if we think the light wave is emitted from the medium of electron's de Broglie wave whose frequency is related to energy perfectly compatible with photoelectric effect.
We often see the (false) explanation that if light is wave. it takes longer time for an electron to absorb energy enough to be ejected from a atom than the case where light is a photon particle in photoelectric effect ( this-4th~5th-paragarphs ).
But actually, electrons in atoms are constantly absorbing various light energies even without the light source ( this-p.2 ).
And electron's de Broglie wave interacting with light spreads over larger area than a single atom, as seen in de Broglie wave interference, which can explain electron's de Broglie wave absorbing light spreading over large area in short time.
Even in the very weak light source, each light is emitted from a single atom, so some small light wave packet emitted from an atom (= Not a photon ) inside weak light can instantly eject an electron in another atom in photoelectric effect.
As a result, classical light wave can perfectly explain photoelectric effect even without quantum mechanical unreal (virtual) photons.
In Compton effect, light wave scattered by an electron and slightly losing its energy is known to elongate its light wavelength.
If light is a photon particle, the photon losing energy must slow down instead of the (non-existent) photon's wavelength being longer (= a rigid photon particle should Not have changeable wavelength, which photon picture disagrees with the observed Compton effect ).
Classical Maxwell equation showed light wave had momentum pressure ( this p.22 ). ← Light wave's momentum and pressure (= based on classical Maxwell theory ) could explain Compton scattering.
As a result, all experimental results including photoelectric and Compton effects proved light is electromagnetic wave, Not a photon particle.
(Fig.27) Photodetectors can detect electrons, Not photons.
In fact, a single photon detector or a photodetector such as avalanche photodiode (= APD ) and photomultiplier can detect only electrons excited by light wave, and can Not detect a (fictional) photon particle itself.
When the incident weak light wave's energy (= E = hf ) and power is above some detection threshold of the photodetector, the light can induce electric current (= flow of excited electrons, Not a photon ), which is (mis)interpreted as a (fictional) photon particle ( this p.2-Fig.1, this-introduction-2nd-paragraph ).
↑ In photoelectric effect, each light needs to have not only frequency but also some minimum intensity enough to eject electrons in photodetector (= light with high frequency with No intensity cannot eject electrons )
The detection threshold of photodetectors can be artificially adjusted as bias voltage (= the bigger the bias voltage, the more electric current is generated by the incident light wave, which was detected as a photon ).
When the light intensity is strong, even the photodetector with low bias voltage (= not only high bias voltage ) can detect the incident light.
This p.8(or p.6)-1st-paragarph says
"for the light counts we see increasing counts as the (reverse) bias voltage is raised. Once
again, we expect this, because a greater bias voltage creates a greater avalanche effect (= more fictional photons are detected as electronic current, this p.3-APD
)"
When the light intensity is very weak, the bias voltage must be increased (= over breakdown voltage, or increasing overvoltage ) to detect the weak light wave as (fictional) photon ( this p.6-Fig.3 shows detecting weak light needs higher (reverse) bias voltage ).
↑ This photodetector is the trick of a single photon's interference where the weak light can split into two weaker lights at two slits, which split lights are too weak to detect at phodetectors simultaneously (= so it looks like only a single photon appears, but actually, the other weaker light, which is too weak to detect at the photodetector, exists in two slit experiments ), and after constructive interference, the final light wave can be detected as (fictional) photon at the photodetector.
↑ So photodetectors just detecting electrons' current excited by (classical) light wave whose power surpasses the detection threshold (= adjustable by bias voltage ) of the photodetectors can explain a (fictional) single photon interference at double slits, entanglement, delayed choice quantum eraser, quantum information even without using unphysical quantum mechanical parallel worlds.
(Fig.28) An orbiting electron does Not lose energy.
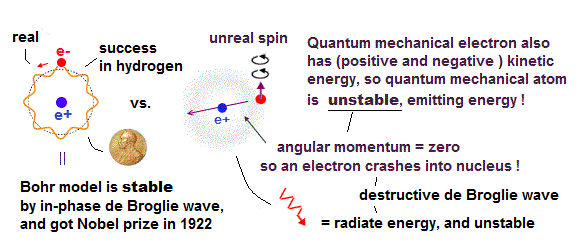
Bohr's realistic atomic model could successfully explain all experimental energy levels of hydrogen-like atoms and ions perfectly ( this last ).
You may often see the (false) cliche "all accelerating electrons radiate and lose energy in classical orbits", so Bohr's hydrogen could be unstable.
This logic is completely unscientific and wrong. So Bohr's orbit is stable, Not radiating energy, as long as an electron is orbiting around a nucleus in a normal orderly way.
To be more specific, "a single accelerated electron ( like in Bohr's hydrogen ) does Not radiate or lose energy."
Only when many electrons are accelerated and colliding with each other in a disorderly way, as seen in alternating currents, they radiate and lose energy.
So the misconception that "accelerating" electron losing energy does Not apply to the successful Bohr model, as long as its electron is moving in an orderly and stable way conserving total energy between a nucleus and an electron, avoiding destructive interference of electron's de Broglie wave.
Actually, Bohr model won the most prestigious Nobel prize, after its scientific legitimacy was admitted as correct by the then academia. ← The misconception that Bohr's atom losing energy was just an excuse made up later to justify unrealistic quantum mechanics.
If the textbook's explanation that every accelerating electron becomes unstable losing energy is right, even quantum mechanical electron which also has kinetic energy (= so quantum mechanical electron is also moving around accelerated by a nucleus ! ) becomes unstable radiating energy. ← self-contradiction.
Quantum mechanical atoms allegedly having unrealistic negative kinetic energies, can never be stable, because its s orbital with zero orbital angular momentum always crashes into a nucleus, and causes destructive interference of de Broglie wave in its linear orbits.
(Fig.29) Bohr model electron is Not falling into nucleus.
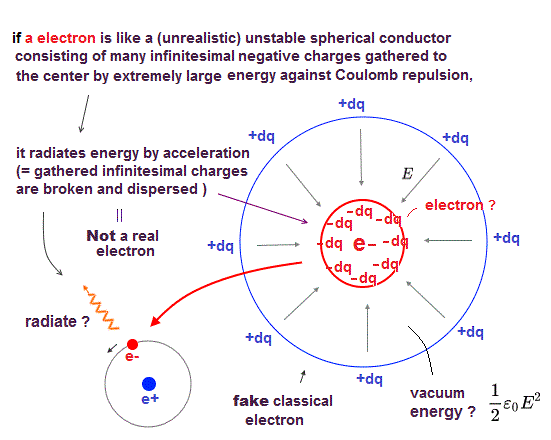
In fact, if a single accelerating electron loses energy, the single electron must consist of many smaller fictitious charges as seen in a spherical conductor in the upper figure.
So Bohr's atomic electron which is an indivisible negative charge does Not radiate or lose energy just by moving around the nucleus in an orderly and periodic way.
The theory of an accelerated charge radiating energy uses "electric energy density field" (= energy = 1/2εE2 ) stored in vacuum around spherical conductor packing many smaller repulsive charges ( this p.4, this p.2 ).
This energy density in electric field equals the amount of energy required to pack many repulsive smaller charges into the same spherical conductor.
So if a single accelerated electron really radiates and loses energy while it orbits around a nucleus, this single electron must be like a fictitious spherical conductor which collects and packs many smaller negative charges into a single electron's tiny body (= whose electric energy density around a fictitious electron consisting of many smaller charges becomes 1/2εE2 ). ← It's impossible and inconsistent with the fact that a single electron is very stable and unbreakable.
A single electron is an indivisible charged particle, which is Not like a spherical conductor packing many smaller illusory negative charges inside a single electron.
As a result, the idea that Bohr's atomic accelerated electron radiating energy is untrue, based on the false assumption.
(Fig.30) ↓ A electron radiates only a virtual photon with imaginary mass !?
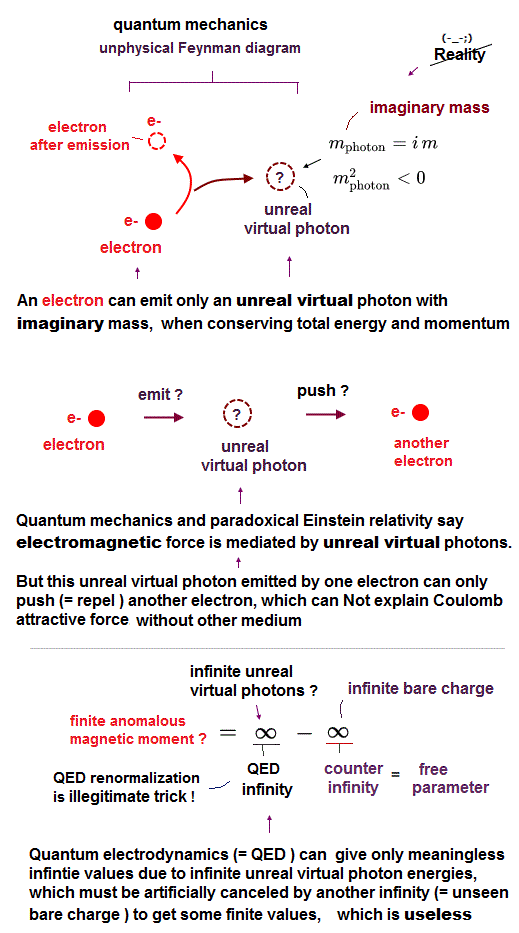
In the current mainstream quantum field theory, all interactions between elementary particles are expressed using unphysical abstract lines called Feynman diagrams which tell us nothing about detailed mechanisms of how particles actually touch or interact with each other.
Quantum mechanics says an electron can interact only with unreal unobservable virtual photons (= Not real photons ! ) with unphysical imaginary mass, when electrons emit, absorb virtual photons, or apply electromagnetic forces to other charged particles ( this p.16, this 6,10 paragraphs ).
Why cannot each electron interact with a real photon (= light ) for explaining light absorption or force interaction according to unrealistic quantum mechanics ( this p.7,8 ) ?
Because the ratios of energy (= E ) to momentum (= p ) are different between an electron and light (= a photon ? ) due to different masses of an electron and a photon (= an electron is much heavier than a photon ).
So if Einstein relativity is right (= if there is no space "medium" mediating forces ), the physics needs unreal virtual particles or virtual photons interacting with real electrons with unreal imaginary masses (= if total energy and momentum are conserved between a electron and an emitted photon ) which violate Einstein's mc2 mass-energy relation ( this p.6, this p.4-last-paragraph, this p.3 ) or violate energy conservation law ( this p.10, this p.3 ) against the fact that all real particles must always conserve total energies and momentums ( this 5th-paragraph, this 5th-paragraph ) !
As shown above, even if an electron can emit a (unreal) virtual photon as electromagnetic force, this emitted virtual photon can only push (= repel ) another photon, which cannot explain Coulomb attractive force (= pull ) without the help of other medium.
Quantum electrodynamics (= QED ) relies on unreal virtual particles or virtual photons with imaginary masses ( this p.17 ), which are said to paradoxically move faster-than-light ( this 1-5th paragraphs, this 9th-paragraph ), which clearly contradicts another mainstream theory of Einstein relativity prohibiting superluminal motion.
↑ The current mainstream quantum field theory includes self-contradiction, so false ( this 10th-paragraph, this p.9-10 ).
Quantum field theory or quantum electrodynamics (= QED ) are just unphysical theory whose unreal virtual particles with impossible imaginary masses ( this p.18 = negative mass squared, this p.2-lower-footnote, ) or negative kinetic energies are also nonphysical math symbols with No concrete physical shapes which pseudo-quantum theory tells us nothing about the detailed physical mechanism, so completely useless and No relation to our real world ( this p.6-lower, this p.13 ).
Actually, No scientists are using this useless quantum field theory for our daily-life application.
↑ The present relativistic quantum field theory and QED requiring unreal virtual particles for explaining physical forces are self-contradictory (= virtual particles contradict Einstein famous relativistic mc2 mass-energy relation, this p.10-11, this p.4-2nd-last-paragraph, this p.4 ), hence wrong theories.
(Fig.37) ↓ Electron, photon are just meaningless math symbols in the useless quantum mechanics.
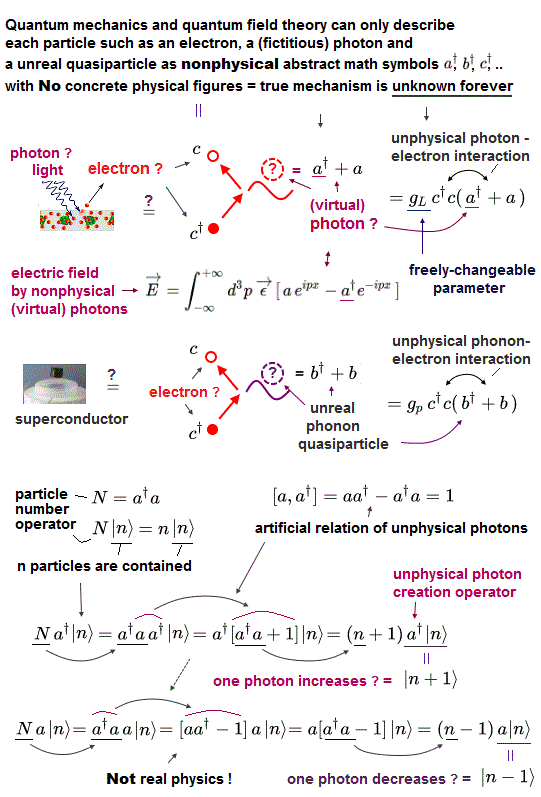
Quantum mechanics, quantum field theory can only express particles and electrons as nonphysical math symbols (= each particle = a†, b†, c†,.. ← No detailed shapes or sizes ) with No concrete physical shapes ( this p.2 ) in all physics such as condensed matter fields, semiconductors ( this p.2 ), superconductors ( this p.3 ), band theories ( this p.2 ) with unreal effective mass ( this-p.2-3, this p.9-right ).
Unscientific quantum mechanics and quantum electrodynamics (= QED ) can also only describe fictitious photon particles as nonphysical meaningless math symbols (= each photon = a†, b†,.. = giving No detailed figures of photons, this p.8, this-p.3-p.8, this p.2-right, this p.16, )
The useless quantum mechanical condensed matter physics has to rely on unreal quasiparticle model such as phonons ( this p.13-lower ) to explain various phenomena such as superconductors by using only the abstract nonphysical interaction term between electrons and unreal phonon quasiparticles (= ex. phonon's creation operator b† ) expressed as meaningless math symbols ( this p.4, this p.6 ).
In these nonphysical quantum mechanical particle description, Pauli exclusion principle is also described as nonphysical math description called anticommutation of nonphysical electron particles, which abstract forms give No detailed physical mechanism of Pauli principle, so completely useless ( this p.8-upper (or p.6 ), this p.7 ).
(Fig.38) ↓ Nonphysical anticommutation relation = Pauli exclusion principle ?
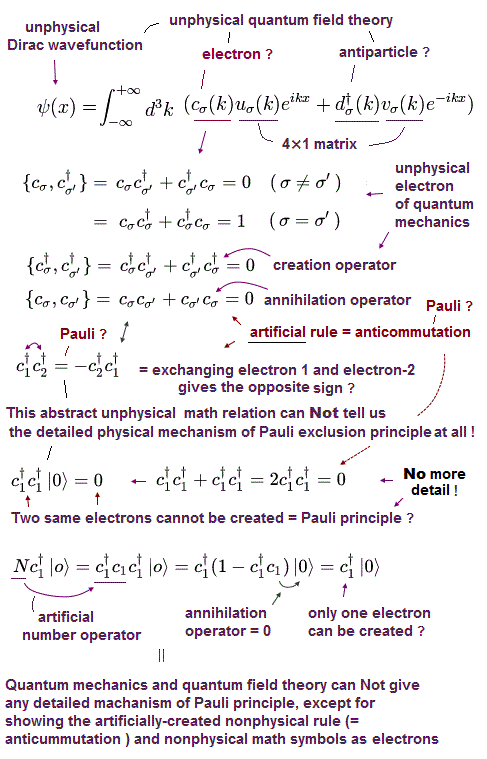
Relativistic quantum mechanics or quantum field theory allegedly combining quantum mechanics and Einstein special relativity becomes more unrealistic and unphysical.
Quantum mechanics refuses to give concrete physical mechanism of Pauli exclusion principle, except for just saying Pauli principle was expressed just as nonphysical antisymmetric wavefunction where exchanging two electrons are supposed to flip the sign of the whole antisymmetric wavefunction (= which was proved to be false ).
In quantum field theory using relativistic Dirac equation which expresses each electron as a mere abstract math symbol, the mechanism of Pauli exclusion principle is also expressed just as meaningless abstract math relation called anticommutation ( this p.3, this p.7, this p.2 upper, this p.5-lower, this p.4, this p.52-53 (or 45-47)-(3.53), this-(8.2.14),(8.2.22), this 3-5th-paragraphs, this p.6 )
In anticommutation relation allegedly denoting Pauli principle, when two electrons (= c1 ↔ c2 ) are exchanged, the sign is changed. ← That's all. No more detailed explanation of Pauli exclusion repulsion is given by quantum mechanics.
↑ So in this abstract quantum field theory's Pauli principle description, if two electron particles (= expressed as nonphysical math symbols c1 and c2 ) are the same ( c1 = c2 ), those electron particles vanish ( c1c2 = - c2c1, → when c1=c2, → c1c1= - c1c1 → 2(c1)2 = 0 → c1 (and c2 ) = 0, = Pauli exclusion principle !? this p.8 or p.6-upper, this p.6, this p.1-2, this p.9 ) ← Nonsense.
What causes Pauli principle is unknown forever, as long as unphysical quantum mechanics continues to be the mainstream theory.
As you see, unscientific quantum mechanics has given up pursuing deeper physical mechanism for a long time, fearing many self-contradictions and unrealistic aspect inherent in quantum mechanics will be exposed, when we start to look into true underlying mechanism.
(Fig.E) All useful things were invented by trial-and-error experiments.
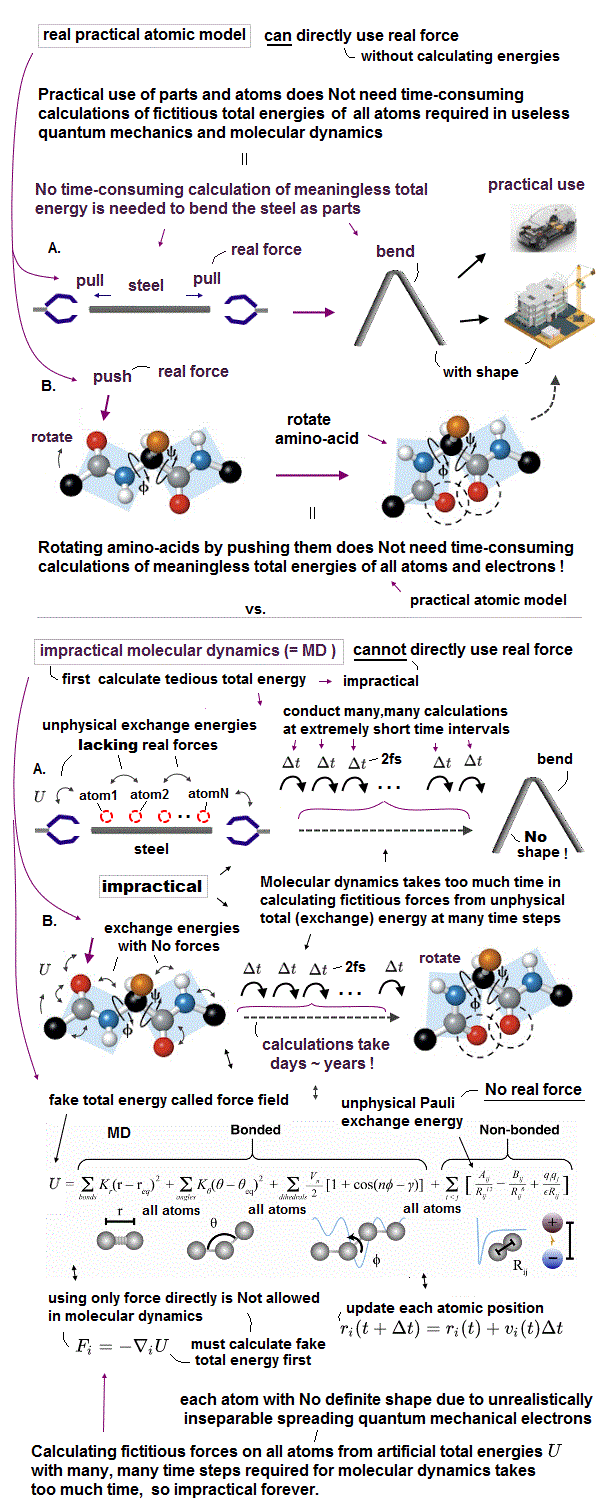
Edison and Wright brothers invented useful light bulbs and airplanes by trial-and-error experiments Not by theories such as unreal quantum mechanics.
Maxwell's electromagnetic theory and Boyle Charles' law were derived from experiments (= so all these basic physical theories are empirical theories ).
Based on these empirical theories, practical radio wave communication and steam engines were invented by trial-and-error experiments.
↑ So theories alone can Not predict detailed experimental values nor invent useful technologies. Only experiments and actual observations can tell detailed values and contribute to technologies.
Basic physical theories such as Newton, Maxwell, Boyle, Charles can just vaguely tell which direction we should advance in development of technologies, cannot tell detailed physical values.
(Fig.Q) Quantum mechanics, QED are unreal, useless, hamper science.
Quantum mechanics uses Schrödinger equation which is useless, cannot predict energies of any multi-electron atoms or molecules ( this-p.21, this-11~13th-paragraphs ).
They have to choose fake trial wavefunctions that may give the lowest (fake) energies out of infinite choices ( this-4th-paragraph ), which takes infinite time, hence, useless, cannot predict experimental energies ( this-p.9-last ).
↑ Physicists cannot know these artificial (fake) energies given by the unsolvable Schrödinger equations are right or not, until they are compared to actual experimental values ( this-p.7-Caveat, this-p.3-last-paragraph ).
↑ So using experimental values from the beginning is OK, much faster, more efficient than wasting too much time in meaningless unsolvable Schrödinger equations.
Quantum mechanical (fake chosen) wavefunctions must be antisymmetric to express unreal Pauli principle exchange energy.
↑ In these unphysical antisymmetric (= exchange ) wavefunctions, each electron must unrealistically spread in all different atoms , which prevent each atom or molecule from having shape, which unreal shapeless quantum mechanism atomic models hamper development of useful technology such as atomic force microscopes.
↑ So the quantum mechanical unreal shapeless atomic wavefunctions cannot even roughly show direction in which we advance in technology (= we cannot even design any molecular machines from the unreal quantum mechanical atomic model lacking atomic shape ).
Because the unrealistic shapeless quantum mechanical atomic wavefunctions forbid us from using even actual atomic shapes obtained from experiments.
Actually, quantum mechanics has to treat the whole molecules and materials as unreal quasiparticle or one-pseudo-electron DFT model lacking real atomic shape.
Transistors were discovered by trial-and-error experiments, Not by (useless) quantum mechanical theoretical prediction ( this-p.16-1st-paragraph, this-p.8-left-2nd-paragraph ).
Quantum electrodynamics (= QED ) always gives meaningless infinities that must be illegitimately removed by renormalization to artificially get some finite values ( this-3~4th-paragraphs ).
This unphysical QED giving only meaningless infinities, which is harshly criticized even by QED founders ( this-1st-paragraph ), cannot predict any physical values, contrary to hypes.
↑ We just use experimental values such as anomalous magnetic moment and Lamb shift from the beginning instead of wasting too much time in this meaningless QED (infinite) calculations.
QED saying unreal virtual photons with imaginary mass ( this-p.16 ) violating Einstein relativity ( this-p.3, this-p.3 ) may mediate electromagnetic force.
↑ These unreal contradictory QED virtual particle models clearly prevent us from describing physical phenomena as real objects, hence, hampers technology.
So quantum mechanics and QED, which are unable to predict anything, are useless, just preventing us from expressing physical phenomena by real objects (= real atomic shape, real charged particle, light medium.. ) and hampering development of technologies.
As a result, we should replace the unreal, useless quantum mechanical shapeless atoms by real simple atomic model with actual shape (= obtained by experiments that are forbidden by the unreasonable quantum mechanics ) to develop practical technologies.
↑ Quantum mechanical unphysical atomic model is not only useless (= unable to predict anything ) but also hampers development of science due to its unreal wavefunction.
The present science focuses only on meaningless illusory theories such as AI that needs experimental data which is hard to obtain due to unrealistic quantum mechanical atomic model.
(Fig.57) Two electrons' de Broglie waves crossing perpendicularly to avoid destructive interference agree with experimental Helium orbits and energies.
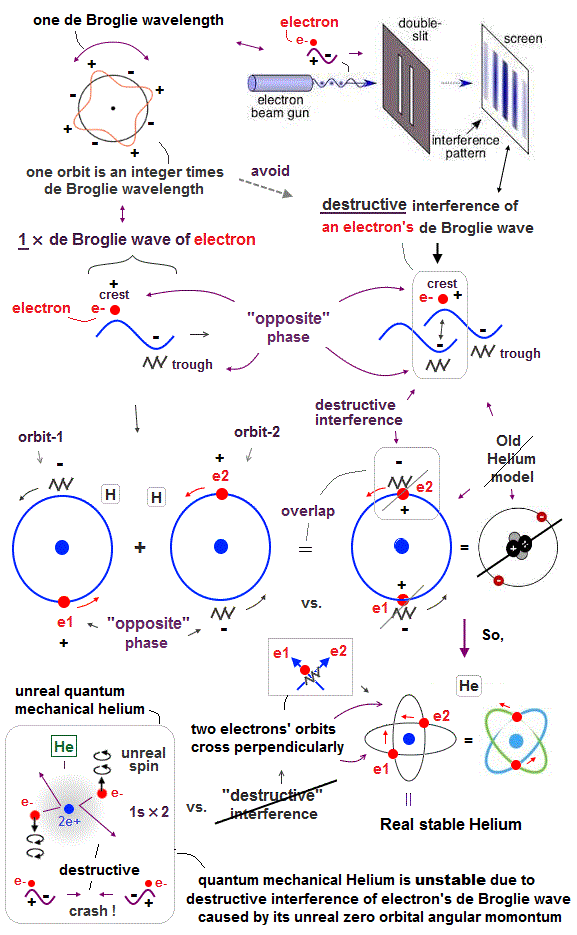
Bohr's atomic model successfully explained energy levels of all hydrogen-like atoms, ions, even fine structure, and obtained Nobel prize. But it could not explain two-electron Helium atom. Why ?
Many experiments showed an electron causes de Broglie wave whose wavelength was determined by observing its interference (= pattern of constructive and destructive interference ).
↑ So we have to naturally consider the electron's de Broglie wave interference (= destructive and constructive interference ) in true atomic electron's orbits.
Realistic Bohr's atomic model could successfully explain the exact energy levels and spectral lines of all hydrogen-like atoms ( this p.2, this p.4, this 3rd-last-paragraph ) by using this de Broglie wave interference (= avoiding destructive interference of the electron's de Broglie wave in its quantized orbit ).
Quantum mechanical unrealistic Schrödinger equations also use this de Broglie wavelength, and agree with Bohr's atomic energies.
But the quantum mechanical unphysical probability wavefunction requires each electron to exist in all different places by using fictional quantum superposition, parallel universes and negative kinetic energy, due to its unrealistic wave-particle duality.
↑ The quantum mechanics must include unreal zero orbital angular momentum where the unrealistic electrons always crash into nuclei in linear orbits, and its unphysical wavefunctions (= representing quantum mechanical de Broglie waves ) are likely to cause destructive interference and become unstable.
↑ Quantum mechanical atomic model (= with its unreal electron spin and parallel-world probability ) contradicts the experimental observations of de Broglie wave destructive and constructive interference (= as seen in the unrealistic linear orbit of zero orbital angular momentum ), so depends on the wrong useless calculation method.
It is impossible for quantum mechanical Schrödinger equations to obtain exact helium atomic orbits and (conserved total) energies, even if it relies on computers, while Bohr's realistic model can get exact helium's orbit and the exact helium's conserved total energy by using a computer that did Not exist in 1920s, as I explain here.
In fact, the unphysical quantum mechanics uses de Broglie wave destructive interference (= as a form of ad-hoc antisymmetric wavefunction ) as the source of Pauli principle repulsive energy or antibond in a wrong way (= because its unreal electron spin magnetic energy is too weak to cause strong Pauli principle repulsion ).
↑ But the unphysical quantum mechanical Pauli principle (= disagreeing with experiments ) is based on contradictory kinetic energy increase through unphysical exchange energy that lacks real Pauli repulsive exchange force or real force carriers ( this p.9-10, this introduction-3rd-paragraph ), requiring each electron to always exist in different atoms, hampering real atomic shape and science.
↑ Quantum mechanical Pauli repulsion based on the unphysical exchange energy or the increased kinetic energy ( this-p.9-10, this-introduction-3rd-paragraph ) contradicts reality, because the real (Pauli) repulsion should decrease the electron's kinetic energy when two repulsive electrons approach each other.
Hydrogen and Helium atoms are the smallest atoms which are known to have up to two 1 × de Broglie wavelength orbits ( which correspond to Schrödinger equation 1s orbital giving the same energies as Bohr model, this p.3-last ).
1 × de Broglie wavelength contains a pair of opposite phases of wave crest (= containing an electron particle ) and trough which contains no electron (= to be correct, de Broglie wave is a kind of longitudinal wave through medium ).
If the opposite wave phases = the crest part and trough part of an electron's de Broglie wave overlap each other out of phase, it causes destructive interference and kicks out the electron from destructive wave, and its electron orbit becomes unstable.
So, when two 1 × de Broglie wavelength electron orbits overlap in the same circular orbit on the same plane (= upper figure, old Helium model ), opposite phases of two electron's de Broglie waves cancel each other, hence, this unstable old circular Helium atomic model is broken and impossible.
This is the consequence of destructive interference between two electrons' de Broglie waves out of phase, which was confirmed in many experiments.
To avoid this cancellation, two electrons' orbits have to be perpendicular to each other, which forms a realistic and stable Helium atom.
This new Helium model with two orbits crossing perpendicularly gives surprisingly accurate experimental energy values not only in a helium atom (= He ) but also in all helium-like ions (= Li+, Be2+, B3+, C4+ .. ← we can obtain exact energy values in these realistic helium perpendicular orbits on the condition that two helium electrons are moving strictly conserving total energy summing Coulomb potential energies and kinetic energies in any positions, and their orbital length is 1 × de Broglie wavelength ), hence, it proves to be a right Helium atomic model.
Unlike the old circular orbit model, the calculation of two perpendicular Helium orbits is impossible by pen and paper. It needs modern computer calculating actual Coulomb force among two electrons and a nucleus and de Broglie wavelength at short time intervals, which could Not be done in 1920s when they chose wrong theory = quantum mechanics.
This destructive interference of real electron's de Broglie wave (= in a right wave different from quantum mechanics ) can naturally explain real Pauli exclusion principle (= 3rd-electron of lithium can not enter 1 × de Broglie wavelength orbit due to destructive interference of real de Broglie waves ) without relying on unphysical quantum mechanical exchange energy (= pseudo-kinetic energy change ).
(Fig.58) Old Bohr's circular helium = electrons are expelled, so wrong.
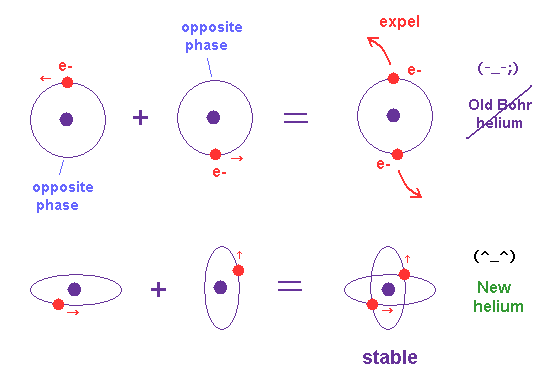
In Old Bohr's circular helium, electrons are kicked out from orbits due to destructive interference between opposite de Broglie wave phases.
Actually, this old Helium model with a single circular orbit containing two electrons gives wrong ground state energy (= -83.33 eV, this-p.2-left-wrong-circular-obit, p.3 table.3-He → He2+ is wrong -83.33 eV ) which is lower than true Helium ground state energy = -79.005 eV = the sum of 1st (= -24.5873 eV ) and 2nd (= -54.4177 eV ) ionization energies of helium.
Two 1 × de Broglie wavelength electron orbits must cross each other perpendicularly to avoid cancellation by destructive interference.
No more electron orbit can enter this new Helium, so it can explain Pauli exclusion principle using real wave or the real repulsive de Broglie wave destructive interference.
(Fig.59) Hydrogen and Helium atoms.
All these orbits are one de Broglie's wavelength.
In this new helium, the two symmetrical orbits crossing perpendicularly are wrapping the whole helium atom completely.
This new helium model is just consistent with the fact of the strong stability and the closed shell property of helium.
Helium atom is known to produce small magnetic field (= Helium has magnetic field called diamagnetic ).
Of course, helium's two orbits are constantly changing their directions, so unable to produce as stably-strong magnetic field as ferromagnet where two neighboring electron orbits in some materials with many orderly aligned atoms are meshing and synchronizing with each other by strong Coulomb force Not by unreal spin.
So Helium has magnetic moment (= spin up + spin down = no magnetic moment ? ) = diamagnetic means quantum mechanical Helium with No angular momentum disagrees with actual Helium producing magnetic moment, so quantum mechanical helium model with unreal spin is wrong and disagrees with experimental facts.
(Fig.60) Two same-shaped orbits are perpendicular to each other.
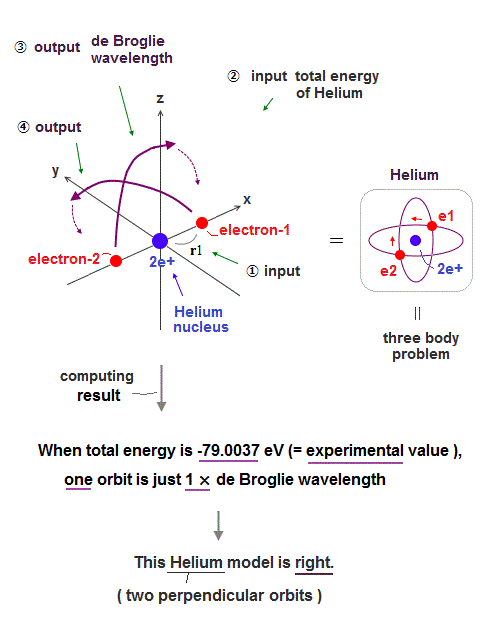
(Fig.61) Right r1 → electron-1 crosses y-axis perpendicularly
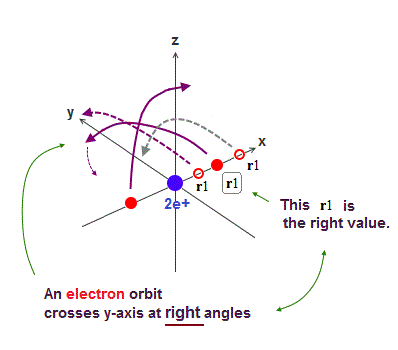
In this computing method, first, we input the initial x-coordinate (= r1 ) of electron-1, which makes the electron-1 start from ( r1, 0, 0 ), and the electron-2's initial position automatically becomes ( -r1, 0, 0 ).
The electron-1 is moving on x-y plane, and the electron-2 is moving on x-z plane (= two orbits are perpendicular to each other ) to avoid the destructive interference of two de Broglie waves.
These two electrons are moving obeying Coulomb force, total energy conservation law strictly, and those electrons' positions, velocities, de Broglie wavelengths are updated at short time intervals, until the electron-1 (or electron-2 ) arrives at the y-axis (or z-axis ).
We can find only one r1 position which makes the electron-1 (or electron-2 ) cross the y-axis (or z-axis ) just perpendicularly (= in this case, the electron's orbit becomes symmetrical around the nucleus, which forms the closed orbit ).
As shown in the calculated results, when the helium's total energy is -79.0037 eV, helium's orbital length becomes just 1.000000 × de Broglie wavelength (= so -79.0037 eV is the predicted helium energy in this calculation ).
The experimentally value of helium total (ground-state) energy is -79.005 eV, so this realistic helium model with two perpendicular orbits perfectly agrees with the experimental energies of the helium atom and all other helium-like ions.
You can choose one of the sample programs of JAVA, simple C, or Python (= all of these three programs give the same results ) below to compute this realistic helium model.
Sample JAVA program
C language program
Python program.
As meter and second are rather large units for measurement of these electron's motion, so we use new convenient units
(Fig.62) New units of time and length.
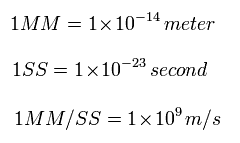
(Fig.63) Acceleration unit
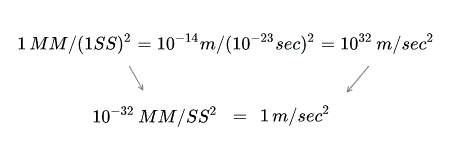
If you copy and paste the above program source code into a text editor, you can easily compile and run this.
When you run this program ( for example, JAVA ) in command prompt, the following sentences are displayed on the screen.
(Fig.64) Example of inputting values in JAVA program
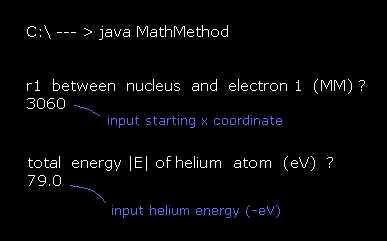
First we input the initial x-coordinate r1 = r (in unit MM ) of electron 1 (see Fig.64 ), and press "enter" key.
For example, in Fig.64, we input "3060", which means the initial x coordinate of electron 1 is 3060 MM = 3060 × 10-14 meter = electron-1's starting point becomes ( 3060 MM, 0, 0 ).
The initial x coordinate of electron 2 becomes -3060 MM, automatically = electron-2's starting point becomes ( -3060MM, 0, 0 ), because two electrons are in two symmetrical perpendicular orbits around the helium nucleus.
Next we input the absolute value of the total energy |E| (in eV) of helium.
In Fig.64, when we input "79.0", and press enter key, it means total energy of this helium is -79.0 eV.
↑ This -79.0 eV is close to actual helium ground state energy = -79.005 eV (= this p.4, this p.9 )
True helium ground state energy is obtained by putting minus to the sum of 1st (= 24.5873 eV ) and 2nd (= 54.4177 eV ) ionization energies of helium = -24.5873 - 54.4177 = -79.005 eV. ← this is experimental value of two-electron helium ground (= the lowest total ) energy.
(Fig.65) Initial states. "r" is initial x coordinate of electron 1.
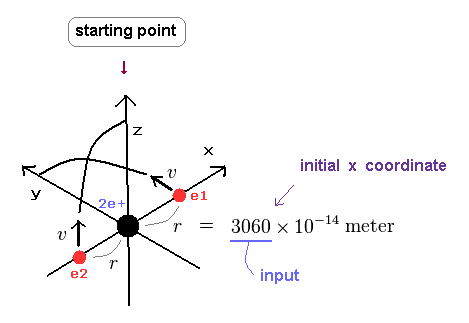
From the inputted values of total energy of helium and initial x-coordinate of electrons (= initial Coulomb potential energy can be obtained ), we can know electrons' initial kinetic energy (= the inputted helium total energy - Coulomb potential energy of the initial electron's positions ) and initial velocity of the electron 1 in y direction (= the initial electron-1's velocity is perpendicular to x axis due to the symmetrical orbit around a nucleus ), which is equal to the electron-2's initial velocity in z direction (= symmetrical to electron-1's orbit ).
For example, initial Coulomb potential energy (= V ) of the initial electrons' state of Fig.65 becomes
(Fig.66) Initial total Coulomb potential energy V.
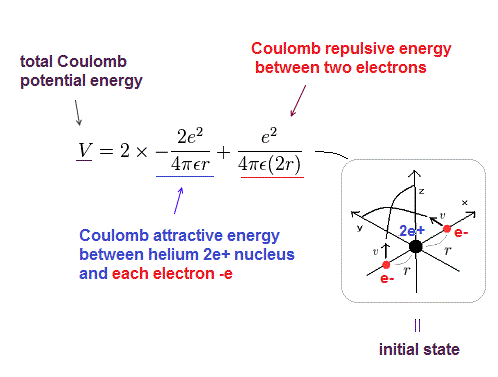
(Fig.67) ↓ Program calculates electron's initial velocity "v" from input values.
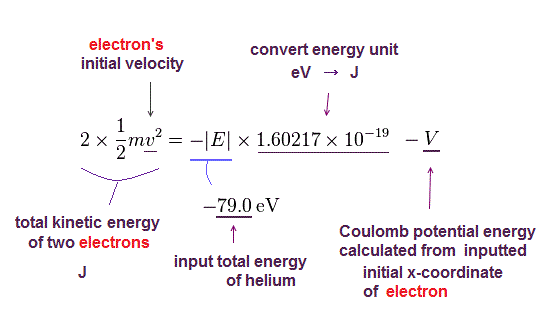
(Fig.69) Positions of two electrons (= perpendicular and symmetric )
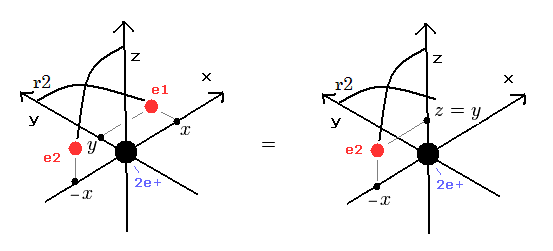
At intervals of 1 SS (= 10-23 seconds ), we compute the Coulomb force among the two electrons and the nucleus based on their positional relationship.
When the electron 1 is at ( x, y, 0 ), the electron 2 is at ( -x, 0, y ) due to their symmetric positions ( see Fig.60 ).
So the x component of the acceleration ( m/sec2 ) of the electron 1 by Coulomb force between electrons and nucleus is,
(Fig.70) x component of electron-1's acceleration by Coulomb force.
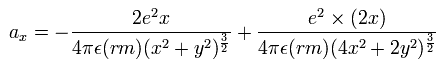
where the first term is the Coulomb force between the nucleus and the electron 1, and the second term is the force between the two electrons.
(rm) is an electron's reduced mass.
(Fig.71) Distances among two electrons and nucleus.
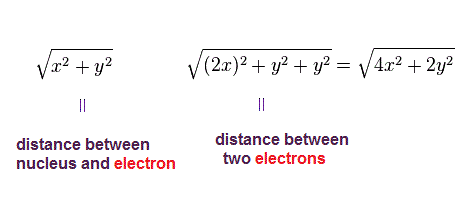
Due to symmetric positions of two electrons, when electron 1 is at ( x, y, 0 ), the electrons 2 is at ( -x, 0, z ), in which z = y.
Due to the finite helium nuclear mass (= Mnuc ), we should use the electron's reduced mass slightly smaller than the original electron's mass (= me ) by considering one stationary nucleus and two moving electrons.
(Fig.72) Reduced mass (= rm ) of one electron.
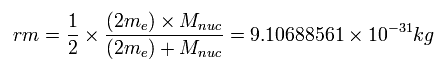
where me is electron's original mass = 9.10938 × 10-31 kg
↑ Helium nucleus (= alpha particle ) is very heavy, so even if we just use the ordinary electron mass (= me ) instead of this reduced mass, this new realistic Bohr model with two perpendicular orbits can get exactly the same right energy as the experimental helium ground state energy.
In the same way, the y component of electron-1's acceleration (m/sec2) by Coulomb force among a nucleus and two electrons is,
(Fig.73) y component of the acceleration by Coulomb.
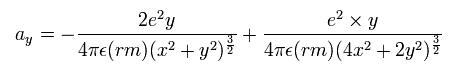
The 1st term of Fig.73 is Coulomb attraction between electron-1 and nucleus, the 2nd term is Coulomb repulsion between two electrons at each position.
Based on calculation of Coulomb force in each position, we slightly change the electron's velocity vector and position at intervals of 1 SS (= 10-23 seconds ) until the electron moves one quarter of its orbit.
(Fig.74) de Broglie waves in each short segment of an orbit.
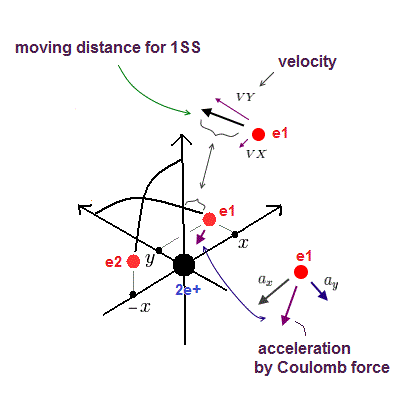
We also calculate de Broglie wavelength (= λ = h/mv ) of the electron from electron's velocity (= v ), mass (= m ) and Planck constant (= h ).
An electron's velocity is gradually changing in its different positions, as the electron is moving in its orbit, so the electron's de Broglie wavelength is also changing in different positions. ← This gradually changing velocity and de Broglie wavelength could Not be calculated in 1920s without computers.
Therefore, we need to divide the electron's orbit into many smaller segments where each short segment equals the distance an electron moves for extremely a short time 1 SS (= 10-23 seconds ).
Then, we compute the acceleration by Coulomb force, change the electron's velocity, obtain de Broglie wavelength and the number of de Broglie wave (= one wave is one de Broglie wavelength ) contained in each short segment at intervals of 1 SS.
The number (= wseg ) of electron's de Broglie waves contained in each short segment (= each short segment is wseg × de Broglie wavelength ) is,
(Fig.75) Number of de Broglie wavelength in the short segment.
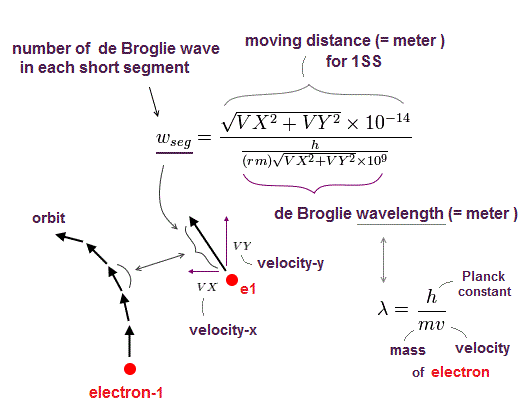
where (VX, VY) are x and y components of electron-1's velocity ( unit 1 MM/SS = 109 meter/sec ), the numerator of the right side of above equation Fig.75 means the moving distance (in meter) for 1 SS. the denominator is electron's de Broglie's wavelength (= h/mv, in meter ).
In the numerator, we change length unit from MM into meter using the relation 1 MM = 10-14 meter.
Here, the estimated electron's orbit is divided into more than one million short segments (= each short segment is moving distance for 1SS ) for this calculation.
When the electron 1 has moved one quarter of its orbit and reached y-axis, this program displays electron-1's last velocity (= VX, VY ) at the last point
(Fig.76) Computing results, when we input 79.0 eV, r1 = 3060 MM. r1 = starting x-coordinate. VX, VY = electron-1's last velocity x, y components after arriving at y-axis. midWN means the number of de Broglie wave contained in 1/4 of the orbit.

After the electon has moved a quarter of the orbit, the program displays the above values on the screen. The initial r1 automatically increases per each calculation of 1/4 orbit.
VX and VY are x and y components of the last velocity of electron 1 ( unit: MM/SS ). ← The last electron-1's velocities (= VX,VY ) are x and y components of the electron-1's last velocities after electrons have moved one quarter of their orbits (= electron-1 should cross y-axis perpendicularly after moving a quarter of its orbit in a symmetrical orbit around a nucleus, so its last VY = velocity in y direction after the electron-1 moves a quarter of its orbit should be close to zero ).
preVY is y component of the last velocity 1ss before VY ( so preVY is almost the same as VY ).
We pick up the values when this last VY is the closest to zero = meaning electron-1 crosses y-axis at right angles, which condition is necessary for the electron-1's orbit to be symmetrical on both sides of y axis around the nucleus.
The symmetrical orbit's total de Broglie wavelength simply becomes 4 × one quarter of de Broglie wavelength.
(mid)WN means the output result of the total number of de Broglie wavelength in one quarter of the orbit. So, one quarter (= 1/4 ) of this electron's orbit becomes WN × de Broglie wavelength.
When one orbit is an integer = 1 × de Broglie wavelength, the 1/4 of the orbit has to be 1/4 (= midWN is close to 0.25000 ) × de Broglie wavelength.
↑ When the output result of one quarter of de Broglie wavelength becomes just 0.250000 (= just 1/4 ), and its input Helium total energy just agrees with experimental Helium total energy (= -79.005 eV ), this new Helium atomic model proves to be right.
(Fig.77) When helium total energy is just -79.0 eV, 1/4 electron's orbit is 0.250006 × de Broglie wavelength. ← so close !
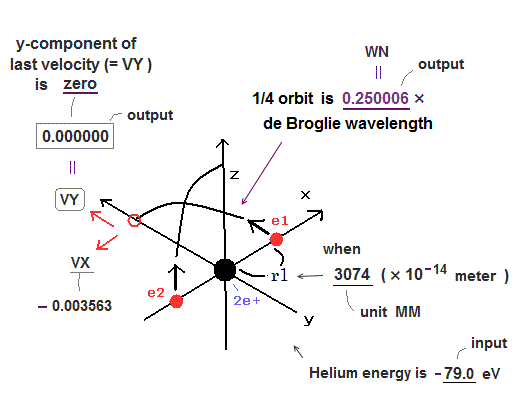
Each time an electron has moved 1/4 orbit and they calculate the total de Broglie wavelength included in the 1/4 of the orbit and the electron's last velocities, this program returns the electron back to the starting x-axis, and increases r1 (= initial x-coordinate of electron-1 ) by 1, until r1 changes from input initial x-coordinate value (ex. 3060 MM ) to +100 (= 3160 ).
As shown in Fig.76, when r1 is 3074 MM, last VY velocity of electron 1 becomes the smallest ( VY = 0.000000 ), which means electron-1 passes y-axis perpendicularly = symmetrical stable orbit.
This means when r1 ( initial x coordinate ) = 3074 × 10-14 meter, these electron's orbits become just symmetric around the nucleus (= so, only this initial x-coordinate 3074 MM value of electron-1 remains as a legitimate one in this case ), and we can know one orbit is 4 × WN (= 0.250006, when energy is -79.0 eV ) = 1.000024 de Brolgie wavelength.
In this case where we input -79.0 eV as helium ground state energy, the number of de Broglie wavelength contained in a quarter of its orbit becomes 0.250006.
So, one orbit is 0.250006 × 4 = 1.000024 de Broglie wavenlength. ( ← so close ! but NOT just 1.000000 )
As shown in Table 1, when input energy is -79.0037 eV, de Broglie wave becomes just 1.000000.
So we can get the final value of -79.0037 eV as calculated ( predicted ) Helium ground state energy, when Helium has two perpendicular orbits of just 1.000000 × de Broglie wavelength.
The experimental value of Helium ground state energy is -79.005 eV (= sum of 1st and 2nd ionization energies of helium ), which just agrees with the computed ( predicted ) energy value of -79.0037 eV, proving this new Helium model is correct.
| Prediction (eV) | r1 (MM) | 1/4 wavelength | one wavelength |
|---|---|---|---|
| -75.00 | 3238.0 | 0.256586 | 1.026344 |
| -78.80 | 3082.0 | 0.250323 | 1.001292 |
| -79.00 | 3074.0 | 0.250006 | 1.000024 |
| -79.003 | 3074.0 | 0.250001 | 1.000004 |
| -79.0037 ← | 3074.0 | 0.250000 | 1.000000 |
| -79.005 | 3074.0 | 0.249998 | 0.999992 |
| -79.01 | 3074.0 | 0.249990 | 0.999960 |
| -79.20 | 3067.0 | 0.249690 | 0.998760 |
| -83.00 | 2927.0 | 0.243907 | 0.975628 |
WN × 4 is the total number of de Broglie's wavelength contained in one round of the orbital.
r1 is the initial electron-1's x-coordinate when the electron-1 crosses y-axis just perpendicularly (= last y-component velocity or VY is the closest to zero )
This computed helium ground state energy value (= predicted energy values by this new helium model ) is -79.0037 eV.
The experimental value of helium ground state energy is -79.005147 eV (= 1st + 2nd ionization energies, Nist, CRC ). ← almost perfect agreement between the prediction and experimental values !
So we can conclude this new Helium model with two electrons' perpendicular orbits giving just the same ground state energy as experimental values, proved to be right.
Surprisingly, all experimental ground state energies of all other two-electron atoms and ions just agree with computed results predicted by this Helium-like model with two perpendicular orbits, proving this new helium atomic model is real.
As a control program, we show the program of hydrogen-like atoms ( H and He+ ) using the same computing method as above. Try these, too.
JAVA program ( H or He+ )
C language ( H or He+ )
Here we use the new unit ( 1 SS = 1 × 10-23 second ) and compute each value at the intervals of 1 SS.
If we change this definition of 1 SS, the calculation results of the total energy (E) in which the orbital length is just one de Broglie's wavelength change as follows,
| 1 SS = ? sec | Result of E(eV) |
|---|---|
| 1 × 10-22 | -79.00540 |
| 1 × 10-23 | -79.00370 |
| 1 × 10-24 | -79.00355 |
| 1 × 10-25 | -79.00350 |
This means that as the orbit becomes more smooth, the calculation values converge to -79.00350 eV.
The programs based on other 1 SS definition is as follows,
Sample JAVA program 1 SS = 1 × 10-25 sec, calculation takes much time.
Old sample JAVA program 1 SS = 1 × 10-22 sec--fast but the results are a little different
(Fig.78) Two-electron Atomic Model ( He, Li+, Be2+, B3+, C4+ ... )
Surprisingly, this new realistic Bohr's helium with two perpendicular orbits perfectly agrees with energies of all other two and three electron atoms ( ions ).
JAVA program ( or C program ) to compute all two-electron atoms or ions. ← After starting this program, we are asked to input atomic number Z of a two-electron atom or ion whose energy we want to calculate.
If you pick Z = 2, this program starts to compute ordinary Helium atom in the same way as above
If you pick Z = 3, this program computes Lithium ion (= Li+ = 3e+ nucleus and two electrons )
If you pick Z = 4, this program computes Beryllium ion (= Be2+ = 4e+ nucleus and two electrons ).
And then we input the initial r1 coordinate of an electron-1 (= MM ), and the absolute value of the total energy (= eV ) like this, which gives the electron-1's last velocity (= pick the zero VY velocity in y direction that is symmetrical orbit around a nucleus ) and number of de Broglie wave contaned in a quarter of the orbit (= midWN = 0.250000 or one orbit of 1 × de Broglie wavelength is the case we want as the predicted energy value ).
| Z | Atoms | r1 (MM) | one wavelength | Predicted results (eV) | Experimental values (eV) | Error (eV) |
|---|---|---|---|---|---|---|
| 2 | He | 3074.0 | 1.000000 | -79.0037 | -79.0051 | 0.001 |
| 3 | Li+ | 1944.5 | 1.000000 | -198.984 | -198.094 | -0.89 |
| 4 | Be2+ | 1422.0 | 1.000000 | -373.470 | -371.615 | -1.85 |
| 5 | B3+ | 1121.0 | 1.000000 | -602.32 | -599.60 | -2.72 |
| 6 | C4+ | 924.85 | 1.000000 | -885.598 | -882.1 | -3.50 |
| 7 | N5+ | 788.0 | 1.000000 | -1223.3 | -1219.1 | -4.20 |
| 8 | O6+ | 685.3 | 1.000000 | -1615.44 | -1610.70 | -4.74 |
| 9 | F7+ | 607.3 | 1.000000 | -2062.0 | -2057.0 | -5.00 |
| 10 | Ne8+ | 544.5 | 1.000000 | -2563.0 | -2558.0 | -5.00 |
| 11 | Na9+ | 493.5 | 1.000000 | -3118.5 | -3113.8 | -4.70 |
| 12 | Mg10+ | 451.5 | 1.000000 | -3728.3 | -3724.47 | -3.83 |
| 13 | Al11+ | 416.0 | 1.000000 | -4392.65 | -4390.1 | -2.53 |
| 14 | Si12+ | 385.7 | 1.000000 | -5111.5 | -5110.8 | -0.70 |
| 15 | P13+ | 359.5 | 1.000000 | -5885.0 | -5886.8 | +1.80 |
| 16 | S14+ | 336.58 | 1.000000 | -6712.7 | -6718.26 | +5.56 |
↑ The experimental ground state energy of each two-electron atom or ion can be obtained from this this or this ionization energies (= the sum of the first and second ionization energies from the right = two largest innermost 1s electrons' energies ).
For example, the experimental ground state energy of Li+ ion with two electrons is the sum of the 2nd (= 75.64 eV ) and 3rd (= 122.454 eV ) ionization energies from this or table, hence -(75.64+122.454) = -198.094 eV.
The experimental total energy value of Be2+ ion is -153.8962-217.71858 (of this-4 Be-CRC, the 1st and 2nd largest ionization energies in 1s orbital on the right ) = -371.615 eV.
↑ For example, after starting this JAVA program ( or C program ), if we input ( atomic number Z = ) 4 (= Be2+ ), the initial r1 coordinate = 1422.0 (= MM = 10-14 m ), the total inonization energy = 373.470 (eV), this program gives the last velocity VY of an electron-1 in y direction = 0 which condition is needed for symmetrical orbits around a nucleus (= there is only one r1 in each given total energy that gives VY = 0, which can test the prediction of this new helium model ), a quarter of its orbit being 0.250000 (= midWN ) × de Broglie wavelength.
↑ So this -373.470 eV is the energy predicted by this new helium-like ion model with two perpendicular orbits (= this total energy gives the symmetrical orbits of just 1.000000 × de Broglie wavelength with the electron-1's last velocity in y direction VY = 0 ), which agrees with the experimental energies of Be2+ ion of -371.615 eV.
↑ For example, when you input the wrong Be2+ ion's total energy |E| = 380.0eV, this program (= Z is input as "4" of Be2+ ) gives the wrong de Broglie wavelength of one quarter of the orbit = midWN = 0.247843 (= 4 × 0.247843 Not equal to 1.000000 ) instead of the right 0.250000 in the input r1 = 1397.5 (= MM ) where last VY = 0.
And this program also gives the wrong energy (= Wrong Ene ) of the old Bohr's atomic model with one circular orbit with 1 × de Broglie wavelength containing two electrons (= Beryllium 2+ ion case ) on the opposite sides of a nucleus, which is -382.660, which disagrees with the experimental value of -371.615 eV.
In C4+ ion case, after running this program, when we input Z = 6 (= C4+ ), (initial) r1 between nucleus and electron 1 = 924.85 (MM), total energy |E| of the two-electron atom = 885.598 (eV), it gives the last VY = 0.000000 and midWN (= de Broglie wavelength of one quarter of an orbit ) = 0.250000, which predicted total energy agrees with the experimental C4+'s total energy = 882.1 eV (= 392.087 + 489.99334 eV of this-6 carbon's sum of 1st and 2nd-largest ionization energies of CRC on the right )
Table 4 shows three-electron atoms and ions such as lithium, Be+, B2+, C3+, N4+ ..
See detailed computing method.
| Atoms | r1 (MM) | one wavelength | Predicted result (eV) | Experimental values (eV) | Error (eV) |
|---|---|---|---|---|---|
| Li | 1949.0 | 1.000000 | -203.033 | -203.480 | 0.47 |
| Be+ | 1427.0 | 1.000000 | -388.785 | -389.826 | 1.04 |
| B2+ | 1125.0 | 1.000000 | -635.965 | -637.531 | 1.56 |
| C3+ | 928.0 | 1.000000 | -944.46 | -946.57 | 2.11 |
| N4+ | 790.5 | 1.000000 | -1314.25 | -1317.01 | 2.76 |
| O5+ | 688.0 | 1.000000 | -1745.70 | -1748.82 | 3.12 |
| F6+ | 609.4 | 1.000000 | -2237.60 | -2242.21 | 4.61 |
| Ne7+ | 546.0 | 1.000000 | -2791.15 | -2797.12 | 5.97 |
Like two-electron atoms or ions, this three-electron atomic program requires the input of initial r1 coordinate of electron-1 and total energy, after approximately fixing the solitary 3rd-electron in 2s outer orbit in the hydrogen-like orbit around (Z-2)e+ nucleus (= Z is the input atomic number, Z = 3 is lithium, Z = 4 is Be+ ion ).
Total energies of the three-electron atoms and ions are given by summing three largest ionization energies (= two 1s electrons + one 2s electron ) on the right side of this-CRC energies.
As shown here, we prove when two electron orbits of 1 × de Broglie wavelength cross each other perpendicularly, they give surprisingly accurate energy results in all two-electron atoms and ions !
(Fig.79) ↓ Helium two electrons e1,e2 orbits must be perpendicular to each other for avoiding destructive interference between two de Broglie waves.
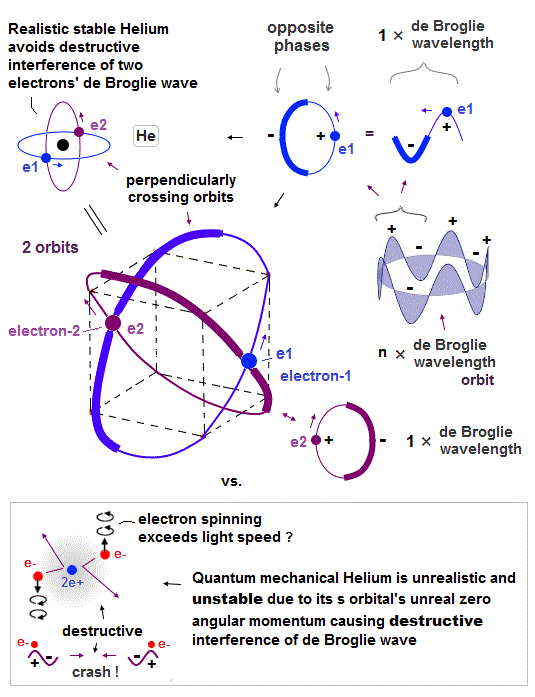
In fact, the unphysical quantum mechanics relies on de Broglie wave destructive interference as the source of Pauli principle repulsive energy or antibond (= its electron spin magnetic energy is too weak to cause strong Pauli principle repulsion ), but it is based on contradictory kinetic energy increase through unphysical exchange energy that lacks real Pauli repulsive exchange force or real force carriers ( this p.9-10, this introduction-3rd-paragraph, this 2. ).
↑ This unrealistic quantum mechanical Pauli principle ( this p.6 ) requiring every electron to exist in all different atomic orbitals simultaneously through the unphysical antisymmetric wavefuntion is the main culprit of hampering technological innovation, disagreeing with experimental results.
So we need to express Pauli repulsion or contact force as "real force" originating from "real substance" to treat atoms and atomic force realistically.
Real electron's de Broglie wave (destructive) interference can be naturally used as the true origin of realistic Pauli principle or its repulsion.
(Fig.80) ↓ Midpoint between de Broglie wave's opposite phases doesn't expel another electron.
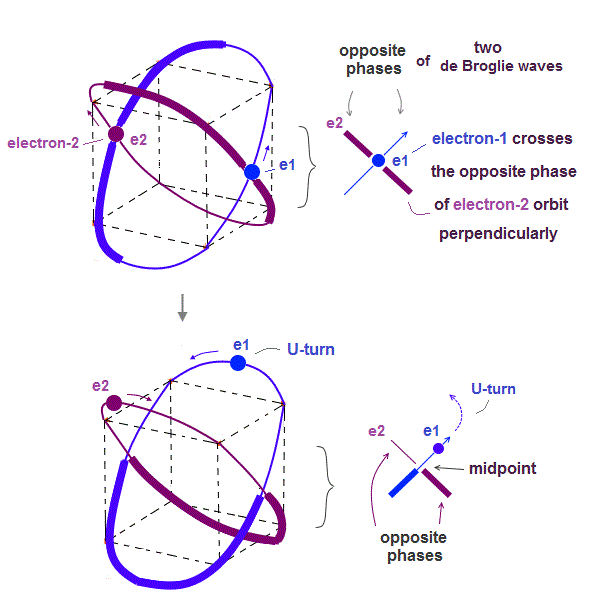
The realistic helium model with two perpendicular orbits avoiding destructive interference of two de Broglie waves can perfectly explain the experimental energies of not only helium atom but also all two-electron helium-like ions.
As shown above, in this realistic helium model, the electron-1 (= e1 ) crossing the other electron-2's (= e2 ) orbit perpendicularly (= perpendicular waves do Not interfere, this p.1-last-paragraph ) can do U-turn safely (= just in the middle point between two crossing points ) when the other e2 orbit is the harmless midpoint (= node ) between ± opposite phases of de Broglie wave.
↑ If the electron does U-turn when the other electron's orbit is the opposite wave phase (= instead of midpoint or the same phase. ← same phases containing electrons are unlikely to approach each other due to Coulomb repulsion between electrons ), the electron is expelled by destructive interference of de Broglie waves, which is real Pauli principle mechanism.
(Fig.81) ↓ An example of real Pauli principle by de Broglie wave's destructive interference. When there are four 1 × wavelength orbits in a atom, an electron is expelled by destructive interference from more than one other de Broglie waves' opposite phase.
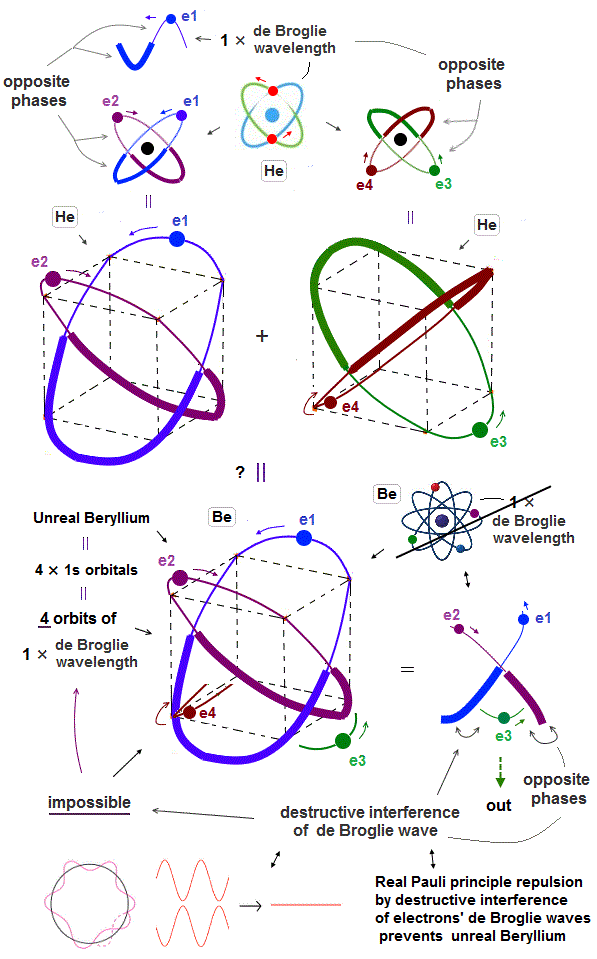
The realistic helium model with two perpendicular orbits avoiding destructive interference perfectly agrees with experimental energies of helium atom and all helium-like ions.
The maximum electron's number in 1 × de Broglie wavelength orbits corresponding to the principal quantum number n = 1 (= remember the quantum mechanical Schrödinger equation also uses de Broglie wave and agrees with Bohr's successful orbits ) is " 2 " as shown in the helium atom (= He ).
Valence electrons of Lithium (= Li ) ~ neon atoms (= Ne ) belong to n = 2 or 2 × de Broglie wavelength orbit (= with larger radius than n = 1 orbit ), as shown in the sudden change of atomic ionization energies ( this 5th-paragraph ).
In an unrealistic Lithium atom with three 1 × de Broglie wavelength orbits (= corresponding to three 1s orbits ), three electrons must be distributed asymmetrically (= so this does Not happen due to asymmetrical Couloum repulsion ) around a nucleus so that these three electrons' orbits cross each other perpendicularly.
↑ In the upper unreal lithium atom, only two orbits of e1 and e2 cross each other's opposite phases perpendicularly.
The e3 orbit crosses e1-orbit's non-opposite wave phases asymmetrically, which cannot maintain "perpendicularly-crossing orbits" by electron-electron repulsion and Not-restricted to perpendicular orbital relationship due to non-opposite wave phases crossing between e1 and e3 (= e3 orbit must cross the same, non-opposite wave phase of e1 orbit before or after the crossing point of e1 and e3 orbits. ).
As a result, this lithium atom consisting of three asymmetrical perpendicular orbits of 1 × de Broglie wavelength is impossible due to real Pauli principle based on de Broglie wave interference.
When there are four 1 × de Broglie wavelength orbits ( like the fictional Beryllium Be atom with four 1 × de Broglie wavelength or four n = 1 orbits ), the electron-3 (= e3 ) is excluded by destructive interference from the opposite phases of two e1, e2 electrons' de Broglie waves ( this-lower ).
↑ In this unrealistic Beryllium with four 1 × de Broglie wavelength orbits, when the electron-3 (= e3 ) passes the middle point between two crossing points with e1 and e2 orbits, this e3 orbit must be perpendicular to both e1 and e3 orbits with opposite phases to avoid destructive interference of de Broglie waves, which is impossible (= each electron can cross only one orbit with the opposite phase perpendicularly. ← One orbit crossing two other orbits perpendicularly at the same time in the same-radius area is impossible ), which is true mechanism of real Pauli exclusion repulsion.
As a result, the maximum electron's number in n = 1 (= 1 × de Broglie wavelength ) orbits is " 2 (= two 1 × de Broglie wavelength orbits, each orbit contains only one electron )" as shown in helium (= He ) atom.
↑ This destructive interference of de Broglie waves is the true mechanism of Pauli exclusion principle based on real repulsive force or real substance (= this real substance or medium has power to cause experimentally-observed electron de Broglie wave interference by pushing out the electron from destructive-interfering area. )
(Fig.82) ↓ 2 × de Broglie wavelength orbit with up to two electrons
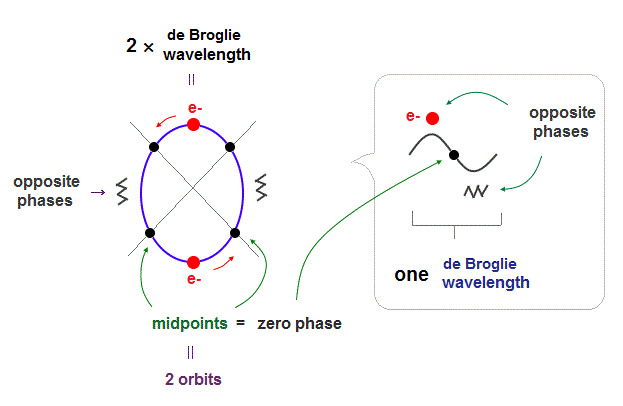
(Fig.83) ↓ Electrons cross the opposite phases of other electron's orbits perpendicularly like Helium without destructive interference.
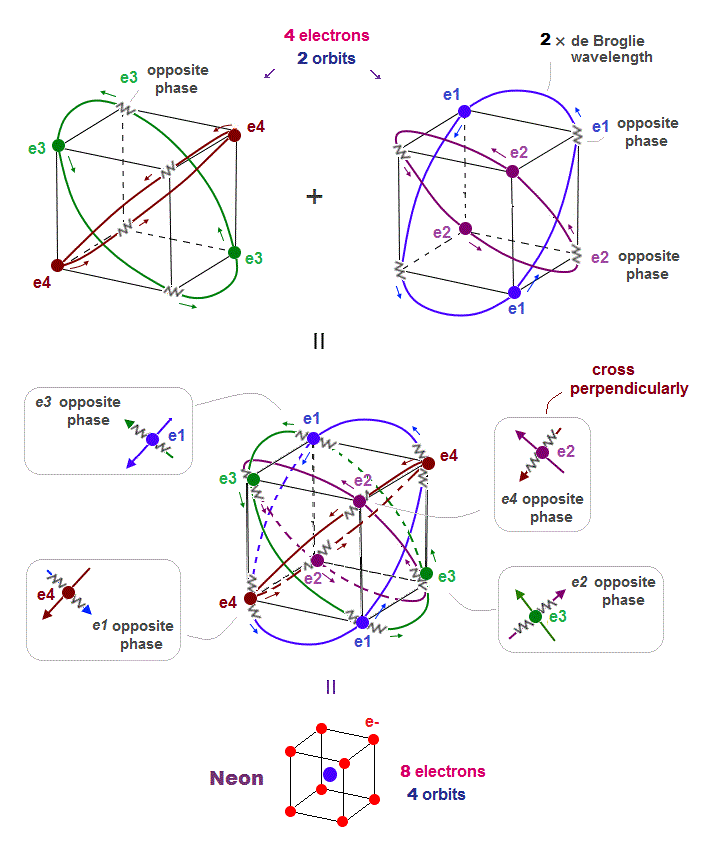
Like Helium, another noble gas = Neon with eight valence (= outer ) electrons can be explained by the same principle based on avoiding destructive interference of other electrons' de Broglie waves.
In periodic table, Neon is in the 2nd horizontal row which corresponds to 2 × de Broglie wavelength orbits in energy levels ( = n = 2 ).
↑ Actually the (unphysical) Schrödinger equation of a hydrogen atom in all energy levels (= related to principal quantum number n ) gives exactly the same energy values as the realistic Bohr's atomic model with n × de Broglie wavelength orbits, because quantum mechanics is also based on de Broglie wave theory, classical Coulomb energy, Bohr radius used in Bohr's atom.
There are clear ionization energy gaps between atoms of different rows in periodic table, which sudden change of ionization energies shows H and He atoms in the 1st row of periodic table belong to 1 × de Broglie wavelength orbits (= n = 1 ), and Li-Ne in the 2nd row belong to 2 × de Broglie wavelength orbits (= n = 2 ).
Neon has eight valence electrons.
And one orbit of 2 × de Broglie wavelength (= two pairs of crest and trough wave phases = two pairs of opposite wave phases ) can contain up to two electrons.
So we can assume Neon consists of four 2 × de Broglie wavelength orbits, each orbit contains two electrons, therefore the total number of Neon valence electrons is 4 orbits × 2 electrons = 8 electrons, which agrees with the fact.
As shown in above figure, all electrons cross the opposite de Broglie wave phase of other electrons perpendicularly at vertices of hexahedron (= this hexahedral Neon structure is Coulomb electrically symmetrical, hence stable electrons' distribution compatible with noble gas ).
So Neon's electrons can avoid destructive interference of de Broglie waves just like Helium.
An orbit of 2 × de Broglie wavelength contains more pairs of wave crest (= electron ) and trough (= opposite phase ) than an orbit of 1 × de Broglie wavelength, so Neon can contain as many as four orbits ( without destructive interference ), such four orbits were impossible in Helium of two 1 × de Broglie wavelength orbits.
(Fig.84) ↓ Real Neon's 8 valence electrons in four perpendicular orbits.
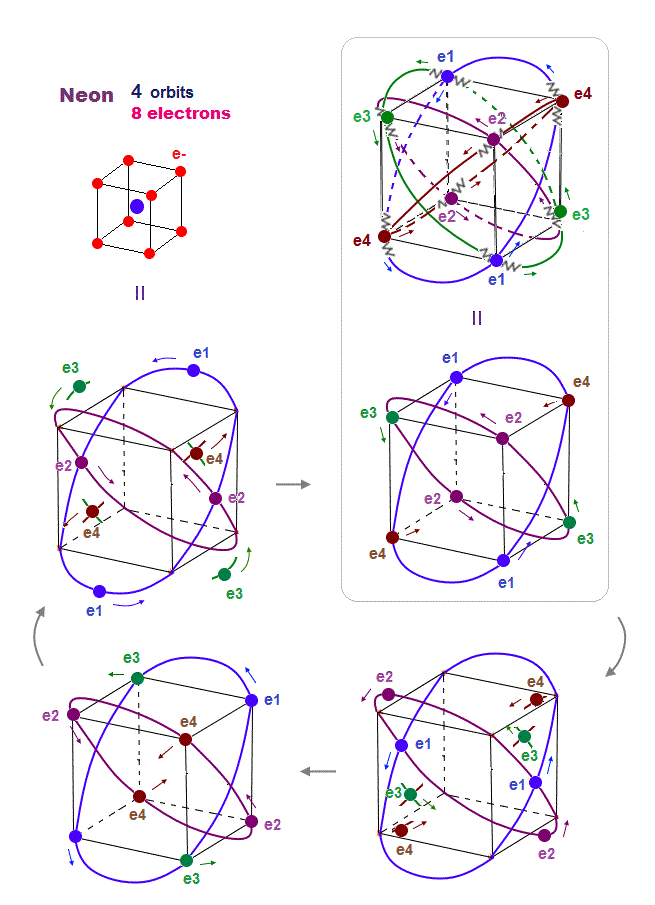
Real Neon consists of four 2 × de Broglie wavelength orbits crossing each other perpendicularly to avoid destructive interference of opposite phases of de Broglie waves.
The upper figure shows how these Neon's 8 valence electrons are actually moving.
(Fig.85) ↓ Electrons safely do a U-turn at midpoints of other electron's de Broglie waves without destructive interference in four 2 × de Broglie wavelength orbits (= each orbit contains up to 2 electrons ).
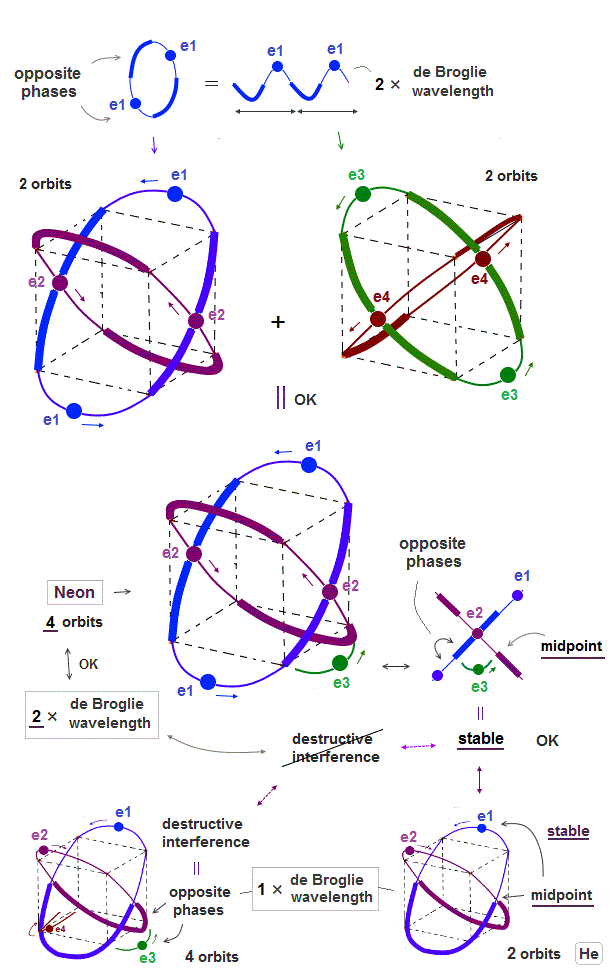
Unlike 1 × de Broglie wavelength of helium (= with only two orbits ), the neon with 2 × de Broglie wavelength orbits can contain four orbits avoiding destructive interference of de Broglie wave.
When the (unreal) atom consists of four 1 × de Broglie wavelength orbits, electrons are unstable and excluded by two other opposite wave phases, when electrons do U-turn between two crossing point, which is real Pauli exclusion principle.
When the (neon) atom consists of four 2 × de Broglie wavelength orbits, electrons can safely do U-turn between two crossing points at the harmless midpoint (= causing No destructive interference ) between opposite phases.
↑ The condition necessary to do the safe U-turn at the harmless midpoint between opposie wave phases is that the distance between two neighboring crossing points of orbits is just 1/2 × de Broglie wavelength, which is why Neon with 2 × de Broglie wavelength orbits can cross more orbits (= total four orbits ) than Helium with 1 × de Broglie wavelength orbits (= total two orbits )
This is why there are only two atoms = hydrogen and helium in n = 1 (= 1 × de Broglie wavelength ) orbits, while there are up to eight atoms = lithium ~ neon in n = 2 (= 2 × de Broglie wavelength ) orbits.
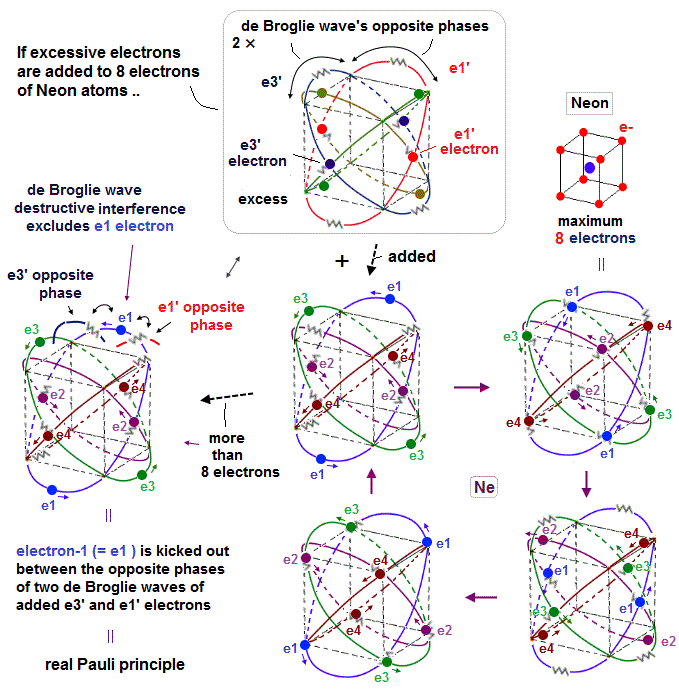
When there are two orbits of 1 × de Broglie wavelength, the electron can do U-turn safely at the harmless midpoint-phase of the other electron's de Broglie wave orbit without causing destructive interference.
But when there are more than two orbits of 1 × de Broglie wavelength, electrons cannot do the safe U-turn at the harmless midpoint (= instead, electrons are always surrounded by the opposite wave phases ), so electrons are excluded by other orbits' opposite de Broglie wave phase through destructive interference.
↑ Due to Coulomb repulsion between electrons, each electron tends to be close to or surrounded by the opposite wave phases of other orbits (= two negative electrons repelling each other in the same phases do not approach each other ), and excluded when there are excessive orbits with respect to de Broglie wavelength (= each electron cannot cross multiple excessive orbits perpendicularly at the same time ), which is real Pauli exclusion principle.
The maximum number of 2 × de Broglie wavelength orbits that can avoid destructive interference is four (= each electron in 8 valence electrons of Neon can do the safe U-turn at the harmless midpoint de Broglie wave phase of other orbits ).
If there are more than four 2 × de Broglie wavelength orbits like in the upper figure-left, the electron (= for example, e1 ) is surrounded by the opposite phase of other orbits (= e3', e1' = Not crossing perpendicularly ), and excluded by destructive interference of de Broglie waves.
If there are excessive electrons, the interval between neighboring intersection points of two orbits becomes less than 1/2 × de Broglie wavelength (= a segment from crest to trough wave phases is the half wavelength ), which causes the opposite wave phases to surround each electron, and destructive interference of de Broglie waves (= real Pauli principle ) occurs.
This destructive interference of de Broglie waves by excessive electrons' opposite phases is the mechanism of real Pauli exclusion principle.
(Fig.87) The opposite de Broglie wave phases cross perpendicularly.
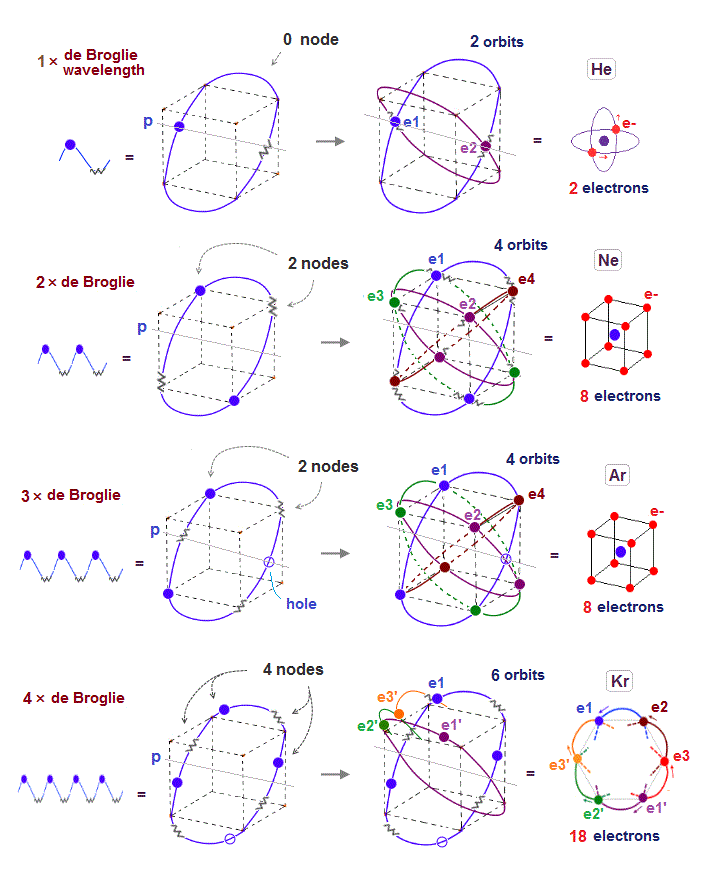
(Fig.88) Maximum numbers of orbits, de Broglie wavelength
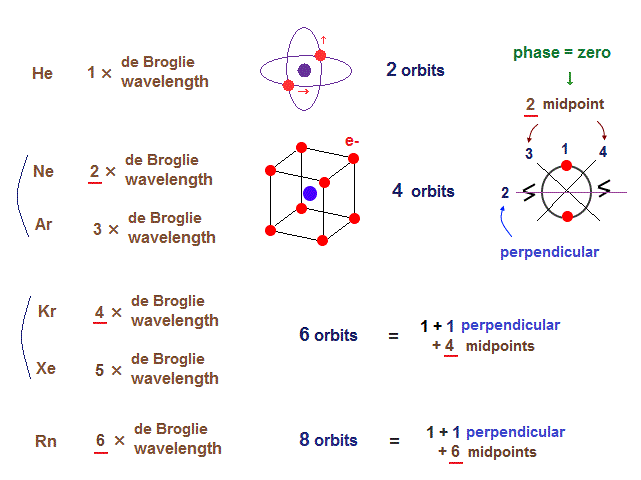
As shown in helium with 2 electrons with two 1 × de Broglie wavelength orbits and neon with 8 valence electrons with four 2 × de Broglie wavelength orbits, de Broglie wavelength determines the maximum numbers of valence electrons by requiring all electrons to cross the opposite phases of other orbits perpendicularly.
Argon (= Ar ) with n = 3 can also have four 3 × de Broglie wavelength orbits containing 8 valence electrons (= Coulomb electrically symmetrical hexahedral distribution ), because each Ar orbit contains two electrons and one hole to avoid unbalanced Coulomb repulsion.
↑ Odd numbers of orbits are unbalanced and unstable with respect to Coulomb electric force among valence electrons, so numbers of stable orbits must be even like 2, 4, 6, 8.. orbits.
Krypton (= Kr ) with n = 4 or 4 × de Broglie wavelength orbits can contain up to 6 orbits for all valence electrons to cross the opposite de Broglie wave phase of other orbits perpendicularly.
Krypton (= Kr ) has 18 valence electrons (= 6 orbits × 3 electrons ), which means each Kr orbit contains 3 electrons and 1 hole to avoid Coulomb electric repulsion like Argon case.
The real numbers of valence electrons can be easily known from periodic table or the change of ionization energies where the change of de Broglie wavelength causes the sudden change of orbital radius or ionization energies.
Xenon (= Xe ) with n = 5 or 5 × de Broglie wavelength orbits also has 18 valence electrons to satisfy the symmetric distribution with respect to Coulomb electric force.
Radon (= Rn ) with n = 6 or 6 × de Broglie wavelength orbits has 32 valence electrons (= 8 × 4 electrons ), which means each Rn orbit contains 4 electrons and 2 holes to satisfy the balanced Coulomb electric repulsion.
↑ So in the stable atomic orbits, the distance between two crossing points must be half (= 1/2 or 0.5 × ) the de Broglie wavelength, which condition is needed for electrons to do the safe U-turn at the harmless midpoint between two crossing points.
If helium (= 1 × de Broglie wavelength ) tries to have more than 2 orbits, or neon (= 2 × de Broglie wavelength ) tries to have more than 4 orbits, or krypton (= 4 × de Broglie wavelength ) tries to have more than 6 orbits, or radon (= 6 × de Broglie wavelength ) tries to have more than 8 orbits, excessive electrons cannot cross the opposite de Broglie wave phases of other orbits perpendicularly, which causes destructive interference and excludes the excessive electrons.
↑ This destructive interference of de Broglie waves is the mechanism of real Pauli exclusion principle not relying on unphysical exchange energy.
Quantum mechanics claiming the maximum numbers of valence electrons obey 2n2 rule disagrees with experimental facts.
So quantum mechanics paradoxically claims 3d orbitals (= n = 3 ) have energies higher than 4s orbitals (= n = 4 ).
↑ Quantum mechanical Schrödinger equations are unsolvable ( this p.7-last-paragraph ) with No ability to predict any atomic energies, so these paradoxical claim like 3d orbital's energy higher than 4s is based on some ad-hoc empirical rules (= like effective Z charge based on Slater empirical rule, this p.2, this 4th-paragraph ) without quantum mechanical prediction ( this p.1-left ).
(Fig.89) If Ar has 12 valence electrons, Coulomb repulsion is Not uniform.
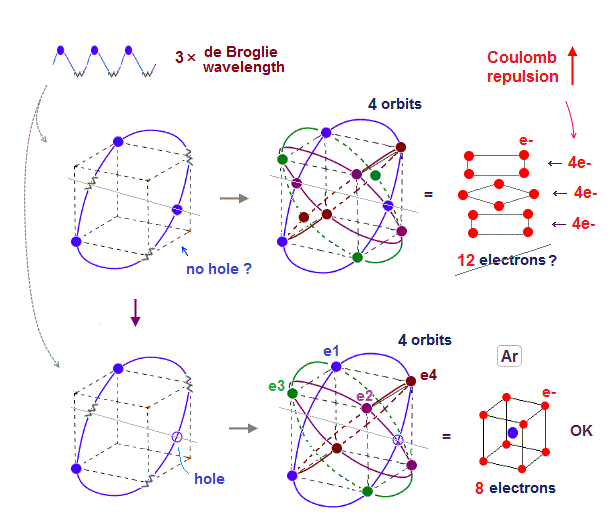
If all 4 orbits of 3 × de Broglie wavelength of Argon (= Ar ) are fully occupied, Argon has 12 valence electrons disagreeing with the actual 8 valence electrons.
As shown in the above figure, this fictitious Argon with 12 valence electrons are unstable due to unbalanced Coulomb repulsive force.
If Argon has 8 valence electrons with 4 orbits (= each 3 × de Broglie wavelength orbit has 2 electrons and 1 hole ), these 8 valence electrons are symmetrically (= hexahedrally ) distributed around the nucleus, which becomes electrically stable.
So we need to consider not only de Broglie wavelength but also Coulomb electric symmetric distribution to determine the valence electron's number.
This is why Argon has only 8 valence electrons.
(Fig.90) Two perpendicular de Broglie wave orbits in H2
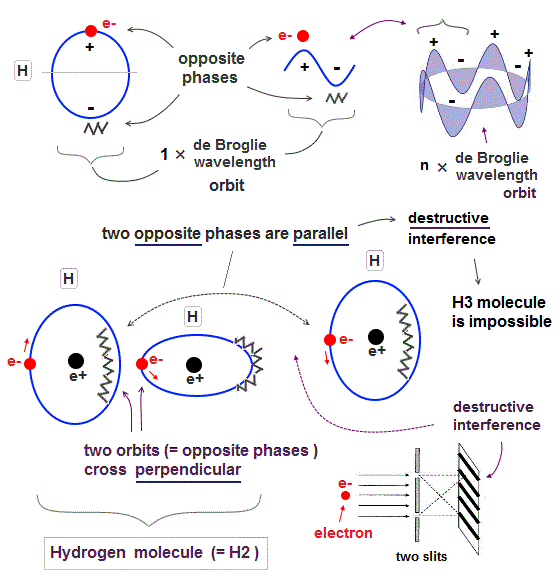
Two orbits (= 1 × de Broglie wavelength ) of the hydrogen molecule (= H2 ) must be perpendicular to each other to avoid destructive interference of electrons' de Broglie wave orbits.
As shown in the upper figure, when the third orbit tries to cross the middle hydrogen orbit perpendicularly, this 3rd orbit becomes parallel to the left hydrogen orbit, and is excluded by destructive interference between opposite phases of de Broglie wave.
Molecular covalent bonds form between electrons and holes of de Broglie wave orbits.
So for example in a oxygen molecule O=O, each oxygen atomic two holes (= each oxygen atom consists of 6 valence electrons and two empty holes ) are always occupied by the other oxygen's valence electrons, so there is No room for more other electrons to approach and form more molecular bonds with O=O molecule.
↑ As a result, this Pauli exclusion principle in molecular bonds is the same mechanism as the atoms, which is restricted by orbits' de Broglie wavelength.
(Fig.91) Wave nature is needed for closed stable orbits ↓
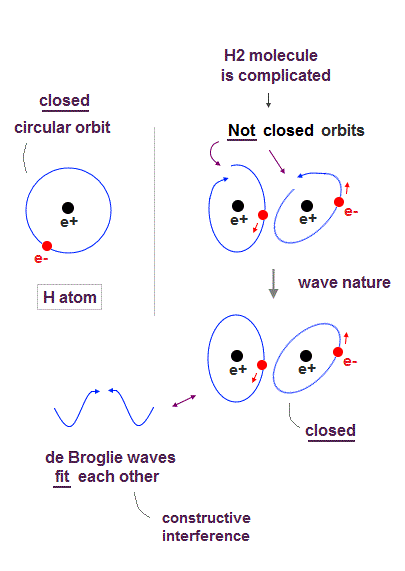
Only in the simplest one-electron hydrogen atom and helium with just 2 symmetrical perpendicular orbits, each electron orbit is naturally closed even if we simply compute the orbits using just Coulomb force (= actually, these real Bohr's helium perpendicular orbits use also destructive interference of two de Broglie waves ).
In all other complicated atoms and molecules, each electron's orbit is usually Not closed, if we consider only Coulomb force for predicting electron's motion.
As shown in two slit experiments, de Broglie wave interference has strong power enough to modify Coulomb force (= an electron is scattered by Coulomb force of slit wall atoms, but the direction in which an electron is scattered is changed by de Broglie wave interference causing fringe. ).
Electron's de Broglie wave tries to avoid destructive interference and synchronize in phase with each other.
Due to constructive interference of de Broglie wave, the electron's orbit is naturally closed (= both ends with the same phase tend to automatically fit and bind to each other for avoiding destructive interference by the pressure balance mechanism where the opposite de Broglie wave phases with low and high density area tend to attract and connect with each other naturally ).
This realistic de Broglie wave interference effect modifying Coulomb force a little needs to be determined by actual experiments. = We should move forward with new experiments by treating electrons, de Broglie waves and Pauli repulsion as real physical objects which realistic atomic model-based scientific progress and experiments are miserably forbidden in the present unrealistic quantum mechanics due to "unrealistic exchange interaction" lacking real physical forces. → Our science stops progressing now.
↑ Even simple hydrogen and helium atoms constantly collide with other atoms and change their directions (= change their orbital shapes ), which needs real de Broglie wave interference power to keep the stable orbits of integer times de Broglie wavelength in addition to Coulomb force.
↑ This de Broglie wave interference effect (= causing each atomic shape and closed stable orbits ) needs to be directly measured by experiments such as multi-probe atomic force microscopes.
Quantum mechanical unreal zero orbital angular momentum causing destructive interference of electron's de Broglie wave and the pardoxical static probability wavefunction cannot explain real de Broglie wave interference nor stable multi-electron orbits, so quantum mechanics is wrong.
↑ As a result, real Pauli exclusion principle and the maximum valence electrons' numbers are determined by destructive interference of de Broglie waves.
(Fig.92) ↓ This realistic H2 molecule proves to be true by computation.
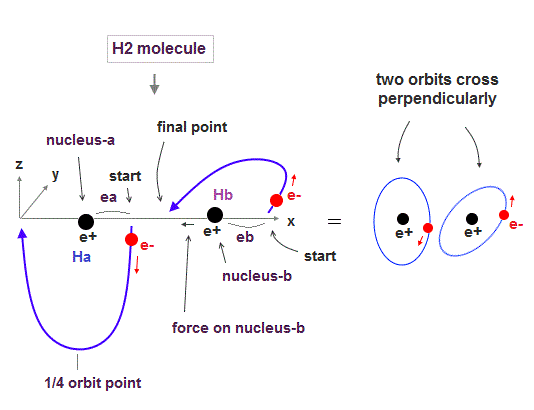
Except for the simplest one-electron hydrogen atom and the helium with two symmetrical (perpendicular) orbits around the same single nucleus, all other aromic and molecular orbits with different multiple nulei or multiple electrons are unable to have the closed orbits (= it's hard for two ends of an orbit to just meet each other on the same point under the complicated multi-electron or multi-nuclear Coulomb forces ), if we simply calculate only Coulomb force.
We need to consider other forces such as de Broglie wave constructive and destructive interference (= which de Broglie wave force or influence must be determined by actual experiments, like Coulomb force relation and electric charge had to be determined by actual experiments at first, a long time ago ) to explain the closed orbits (= de Broglie wave's lower and higher pressure regiones attract each other, and form an integral multiple of de Broglie wavelength by constructive interference ) in more complicated multi-electron atoms and molecules.
So we need to use the approximate method of estimating real H2 molecular orbits using the following computing program, and prove that realistic Coulomb force and de Broglie wavelength can reasonably explain the actual hydrogen H2 molecular experimental bond energy, even without relying on the contradictory unrealistic exchange energies.
Two orbits of H2 molecule are supposed to be perpendicular to each other. Here we suppose electron-a (= or electron-1 ) moves in the orbit parallel to x-z plane, and electron-b (= or electron-2 ) moves in x-y orbit, as shown in Fig.92.
Sample JAVA program to compute H2 molecule (= two perpendicular orbits ).
When you run this program, you need to input the initial x-coordinate of electron-a (= ea ) and electron-b (= eb = distance between nucleus-b and electron-b ) in the unit of MM ( 1MM = 10-14 meter ).
Then, you are asked to input the absolute value of the binding energy of H2 molecule (= experimental value of H2 binding energy is 4.746 eV, this p.2 ).
Lastly, you are asked to input the distance between two nuclei of H2 molecule (= experimental value of H2 internuclear distance is 0.7414 Å ).
From these 4 input values, this program calculates two electrons' motions and orbits based on Coulomb electric force (= calculate Coulomb force and de Broglie wavelebgth, change the electrons' positions and velocities at short time intervals, until an electron moves a half orbit ), and outputs de Broglie wavelength (= a value closer to "1" × de Broglie wavelength is better ) in one orbit, final coordinates of two electrons, and average forces acting on two nuclei (= for H2 nuclei to keep staying in the same positions, average forces acting on nuclei need to be close to zero ).
As I said, each H2 molecular orbit cannot be closed, only if we simply calculate it.
So we approximately suppose these two electrons experience the same average Coulomb force (= summing Coulomb force acting on two electrons and divide it by 2 at each electron's position at short-time intervals ), and two electrons have approxiamtely the same average velocity in two (perpendicular) symmetrical orbits.
↑ So this approximate method tends to give the lower binding energy (= higher H2 total energy ) than the actual H2 binding energy without approximation, but can give a pretty good result close to reality.
For example, we input the initial electron-a x-coordinate = ea (= "3370" MM = 0.3371 Å, here this unit is used, so input "3370" ), the initial electron-b's x-coordinate eb (= "4938.5" MM ), binding energy (= "4.746" eV = experimental value ) and distance between two nuclei (= "7414" MM = experimental value ).
In this case, the calculation results show one orbit of each H2 orbits is 0.98488 × de Broglie wavelength, which is almost an integer = "1" × de Broglie wavelength orbit, so this good result proves the realistic H2 molecule model with two perpendicular orbits is right (= as I said, this "approximate" method tends to give lower bond energy, so this de Broglie wavelength result tends to be slightly smaller than 1.00, which is a reasonable result ).
And in this case, average forces acting on nucleus-a is -0.000082 ( here, the force between electron and proton separated by Bohr radius is supposed to be "1" ) which is almost 0, meaning nucleus-a of this H2 molecule is almost stationary and stable at the same position surrounded by these H2 realistic electrons' orbits.
In the same way, the force acting on nucleus-b is also almost 0 (= -0.000128, The sign = "plus+" force is in the direction of the other nucleus ), meaning nucleus-b is also stationary and stable at the same position in this realistic H2 molecule.
So this realistic hydrogen molecule model with two perpendicular orbits of almost 1 × de Broglie wavelength just agrees with experimental values of H2 binding energy (= 4.746 eV ) and internuclear distance (= 0.7414 Å ).
Here we calculate another case when two orbits of H2 molecule is on the same plane instead of perpendicular to each other.
↑ When two electrons are on the same plane (= neglecting de Broglie wave destructive interference causing realistic Pauli repulsion ), two electrons can avoid each other more, lower the total energy, and its H2 bond energy becomes bigger than two perpendicular orbits.
Sample JAVA program to compute H2 molecule (= two orbits on the same plane ).
After running this program (= compile it with the file name of "hhmol.java" ), when we input the initial electron-1's x-coordinate ea = 3250 MM (= 3250 × 10-14 meter ), the initial electron-2's x-coordinate eb = 5570 MM, H2 bond energy = 4.746 eV (= experimental valeu), and the H2 internuclear length (= 7414 MM = experimental value ).
The result shows the one orbit is 1.001982, which is slightly bigger than the integer = 1.00 de Broglie wavelength (= when average forces acting on two nuclei become almost zero = stable nuclei ).
↑ This result shows when we consider two H2 orbits on the same plane, its total energy becomes slightly lower and its bond energy becomes bigger then the experimental values (= if we try to choose the case giving an integer 1 de Brolgie wavelength instead of 1.001982, its bond energy is bigger than the experimental value of 4.746 eV ).
This means the actual H2 molecular two orbits are perpendicular to each other by the realistic Pauli principle based on de Broglie wave interference instead of two orbits on the same plane.
Here we use the rough approximation supposing two electrons of H2 molecule always have the same velocity and experience the same Coulomb force (= average of two electrons ), so the upper 0.98488 de Broglie wavelength of two approximate perpendicular orbits become closer to an integer "1" with non-approximate method (= as I said, this truly non-approximate method needs to consider de Broglie wave interference's effect, which must be determined by experiments, in order to give closed orbits ).
These calculation results show that the realistic Coulomb attractive energies are strong enough to give the experimental values of H2 molecular bond energy (= unrealistic quantum mechanical exchange energy is unneeded ), and the actual H2 molecule orbits are perpendicular to each other (= proving the realistic Pauli principle ) instead of two electron orbits existing on the same place.
(Fig.93) ↓ Quantum mechanics uses unreal quasi-particle without force.
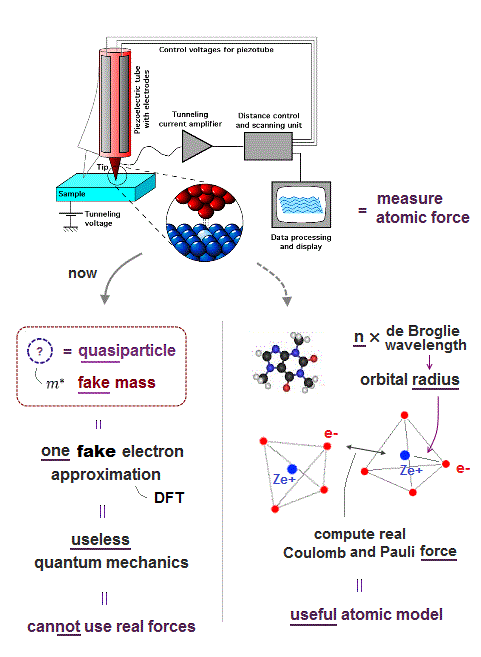
Atomic physics stops progressing in quantum mechanics and molecular dynamics.
We havet to give concrete shape to each atom like macroscopic objects to utilize atoms for technological innovation.
♦ Valence electrons
♦ Bohr's Neon,
Carbon bonds,
Four-fundamental forces.
♦ de Broglie waves determine all atomic structures.
♦ Truth of electromagnetic waves.
♦ others atomic size.
Japanese version

2020/ 12/17 updated. Feel free to link to this site.