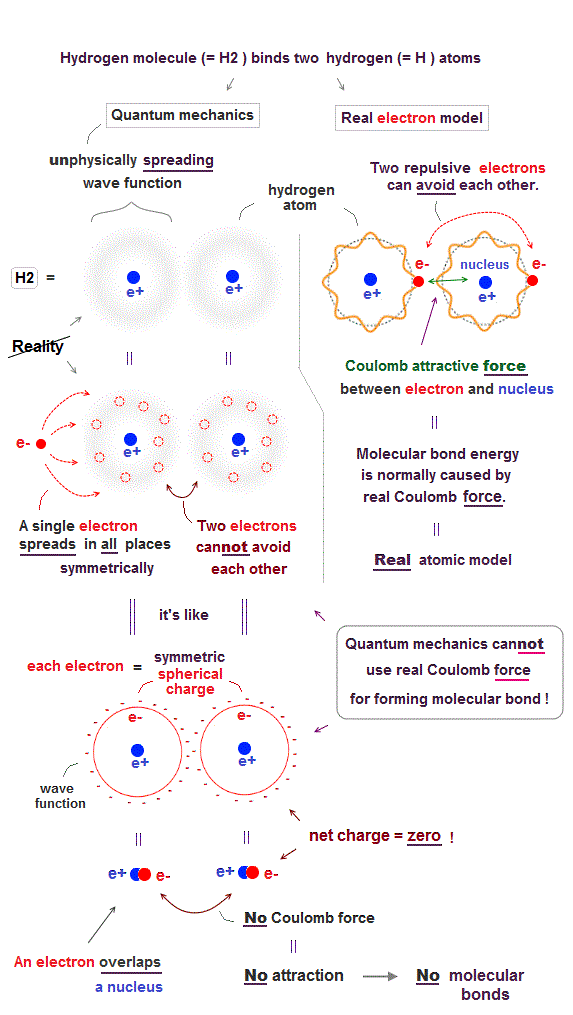
(Fig.1) ↓ No Coulomb force between two quantum wavefunctions.

In fact, quantum mechanical unphysical probability wavefunction is unable to use ordinary Coulomb electric force for forming covalent molecular bonds.
As shown in the upper figure-right, in the realistic atomic orbit such as successful Bohr's atom, an electron is actually moving around, approaching the other positive nucleus and avoiding the other negative electron to form stable molecular bond by ordinary Coulomb electric force.
On the other hand, quantum mechanical unphysical probability wavefunction or electron cloud must always spread out symmetrically around the nucleus to infinity by fantasy superposition or parallel universes.
This symmetrically-spreading quantum mechanical wavefunction or electron cloud is like the symmetrical spherical charge which average electron's position is at the same position as the central nucleus, which generates the zero net charge by the negative electron and positive nucleus canceling each other.
If the quantum mechanical spreading wavefunction tries to shrink only to one position, the localized shrunk wavefunction or de Broglie wave becomes sharper (= de Broglie wavelength is shorter ), and the electron's kinetic energy drastically increases, which disagrees with experimental atomic energies.
As a result, the unphysical quantum mechanical wavefunction, which cannot use the real Coulomb force for forming molecular bonds, has to rely on another unphysical energy called exchange energy allegedly caused by lower kinetic energy.
(Fig.2) ↓ An electron-1 must exist in two different H atoms-A and B simultaneously.
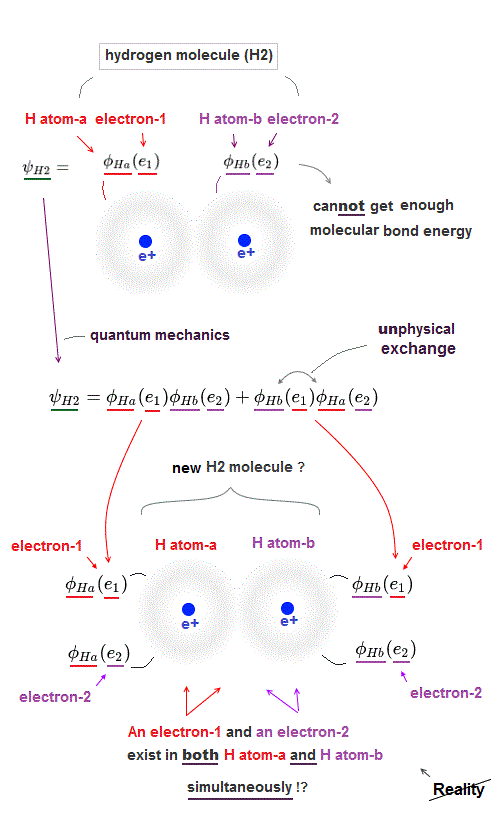
For unphysical quantum mechanical molecules to generate covalent molecular bond energy (or strong Pauli repulsive energy ), each quantum mechanical electron must exist in all different atoms in a molecule simultaneously, which prevents each atom from having its shape nor boundary.
For example, in the upper hydrogen H2 molecule consisting of two H atoms-A and B, both the electron-1 and electron-2 must exist in two different H atoms-A and B simultaneously ( this p.3, this p.11, this p.4 ).
φA ( r1 ) means the electron-1 existing in H-atomic wavefunction φA.
φB ( r1 ) means the electron-1 existing in H-atomic wavefunction φB ( this p.18 ).
H2 molecular Hamiltonian H or total energy consists of two electrons' kinetic energy expressed as space derivative (= de Broglie wave ) and Coulomb electric energy terms among two H atomic nuclei-A, B and two electrons-1, 2, as shown above.
(Fig.3) Real molecular bond attraction should increase electrons' kinetic energy, which contradicts the unreal quantum mechanical molecualr bond exchange energy based on decreased kinetic enregy
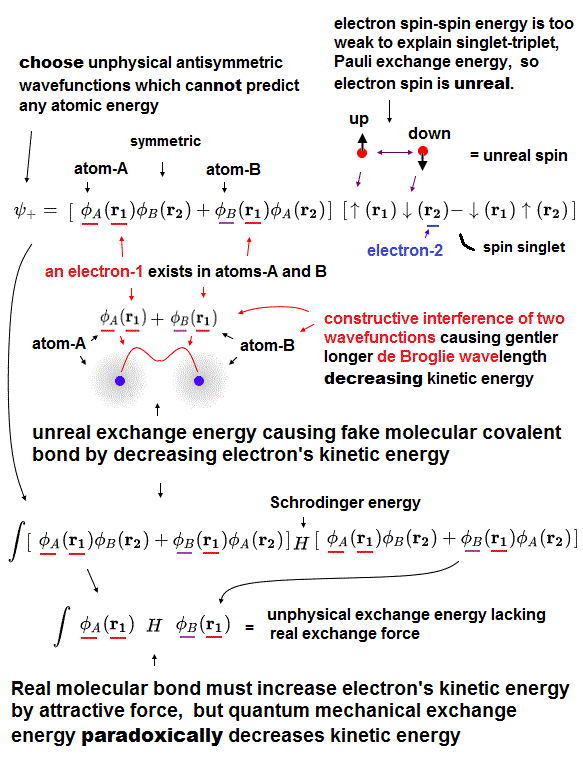
Quantum mechanical Schrodinger equations are useless, unsolvable, unable to predict energies for atoms or molecules.
↑ They have to artificially choose fake trial wavefunctions with many freely-adjustable parameters to get (fake) atomic or molecular energies close to actual values, which quantum mechanical ad-hoc time-consuming method cannot predict atomic energies.
Physicists have to artificially choose the unphysical antisymmetric wavefunctions as these fake trial wavefunctions to express the unphysical Pauli exchange energies (= artificial chosen quantum mechanical wavefunctions cannot predict Paili exchange energy ).
Quantum mechanics unrealistically demands each electron-1 and 2 must exist in two different H atoms-A and B to form fictitious H2 molecular covalent bonds in the artificially-chosen fake antisymmetric wavefunction for unsolvable Schrodinger equations.
To form molecular attractive covalent bonds, symmetric wavefunctions (= spin part is up-down singlet antisymmetric or bonding ) are necessary ( this p.3-4 ).
↑ But electron spin-spin magnetic energy is too weak (= just 0.0001 eV, this-p.10-left-2nd-paragraph ) to cause singlet-triplet exchange energy splitting (> 1eV, this p.3-middle, this 3s3p singlet 1p and triplet 3p energy difference is 13140cm-1 = 1.629 eV ) or Pauli exchange energy (> 10eV ), so electron spin is unreal, unnecessary.
By putting H2 molecular ( Schrodinger equation's ) total energy Hamiltonian (= H ) between these (artificially-chosen) two symmetric wavefunctions (= ψ+ ) where each electron-1 and 2 exists in two different H atoms-A and B and integrate them, we can get two different integrals called Coulomb energy integral and (unphysical) exchange energy integral ( this p.3-4, this p.14 ).
Coulomb energy integral is the normal integral where each electron-1 (or electron-2 ) consists of two same H atomic wavefunctions like ∫φA(r1)φA(r1) and ∫φB(r1)φB(r1).
Unphysical exchange (= resonance ) energy integral consists of two different H atomic wavefunctions like ∫φA(r1)φB(r1) and ∫φB(r1)φA(r1).
This unphysical exchange energy integral is necessary to cause fictitious quantum mechanical molecular bonds (or Pauli repulsive antibonding ), and the ordinary Coulomb energy (integral) has No power to form molecular attractive bonds ( this p.3, this-lower-Fig.2, this p.4-5, this p.4-5-Fig.1 ) or Pauli repulsive antibonding.
This (or this )-p.3-last~p.4 say
"and J and K are Coulomb
and exchange integrals"
"but the contribution of J (= Coulomb energy ) is far smaller (in absolute value)
than that of K (= unphysical exchange energy integral )"
In this symmetric wavefunction (= constructive interference of two wavefunctions ), the total wavefunction becomes gentler, which means the total de Broglie wavelength becomes longer, and electron's kinetic energy decreases ( this-p.12-conclusion ).
This decrease in electron's kinetic energy is the driving force of forming molecular attractive covalent bonds caused by unphysical exchange energy.
This-p.1-1st-paragraph says
"chemical bond formation is driven
by a decrease in electron kinetic energy."
We often see the wrong explanation that higher electron's density between two H atoms may cause decrease in Coulomb electric energy and molecular attractive bonds.
But even if Coulomb potential energy decreases, kinetic energy increases to conserve the total energy, the molecular attractive bonds cannot be formed, so the electron's lower kinetic energy even in the lower Coulomb potential energy region is the reason of quantum mechanical unphysical exchange molecular bonds.
This p.4-left-last-paragraph~right says
"Many textbooks are saying that chemical bonding is a result of the accumulation of density in the binding
region. This is a deceptive statement, because the origin for
the charge accumulation is Not given"
"This author showed that the kinetic energy plays a paradoxical role in the chemical bond formation"
"Ruedenberg has shown that it is actually a decrease of T (= kinetic energy ) which accompanies the charge accumulation in the bonding region"
This quantum mechanical molecular bond exchange energy based on lower kinetic energy is unphysical and wrong ( this p.11 ).
Because if the real attractive force is working between two atoms, electrons are attracted and accelerated toward the other atoms, so electrons' kinetic energies should increase instead of decrease (= when two atoms repeling each other, electrons decelerate, and kinetic energy should decrease ).
↑ Quantum mechanical exchange energy based on kinetic energy violates basic physical principle, so wrong.
This contradictory quantum mechanical exchange energy due to kinetic energy change lacks real exchange force or physical reality ( this p.9-upper, this p.8-last, this p.5, this p.8-right-Discussion ).
(Fig.4) Even when an electron-1 approaches the other H-atom-B's positive nucleus decreasing Coulomb potential energy, its kinetic energy keeps decreasing, violating energy conservation = trick of unphysical exchange energy.
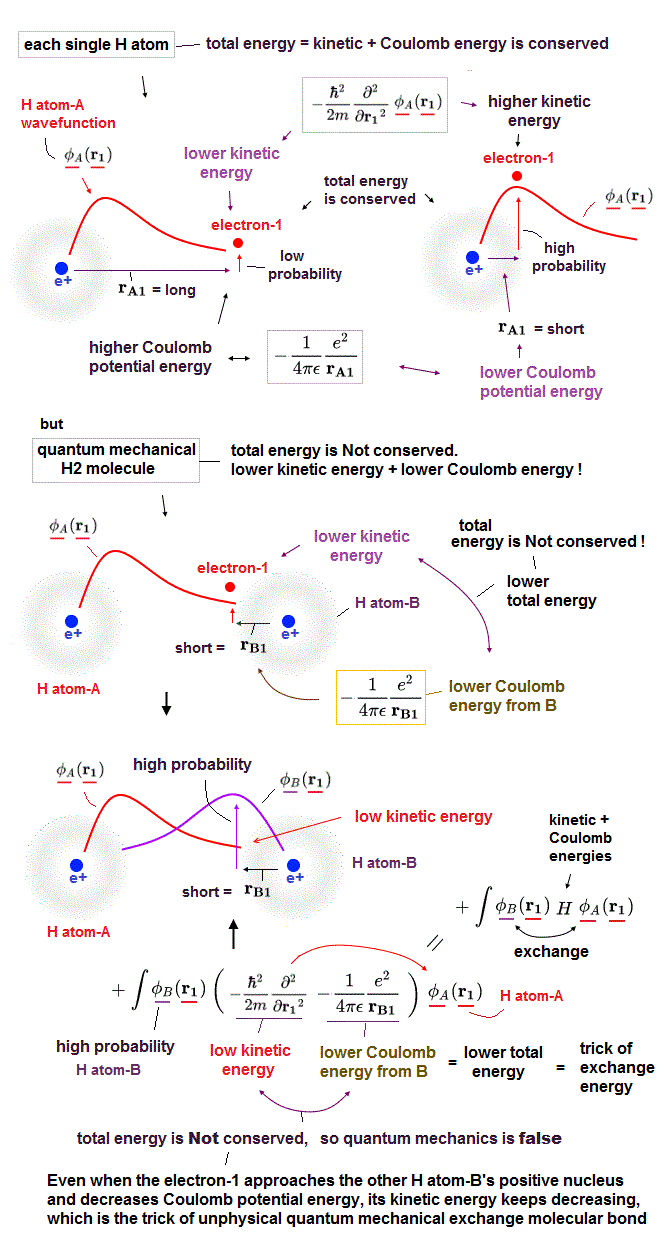
No quantum mechanical Schrödinger equations are solvable except for one-electron hydrogen (= H ) atom ( this p.21-upper ).
In only this solvable H atom, the total energy E, which is the sum of electron's kinetic energy expressed as derivative and Coulomb potential energy, is always constant and conserved in any electron's positions ( this-p.2-upper, this p.2-1st-paragraph ).
So in H atom, as the electron-1 is farther away from the H atom-A's positive nucleus, it increases Coulomb potential energy and decreases electron's kinetic energy to keep the constant and conserved total energy E.
In molecules such as hydrogen molecule (= H2 ) and hydrogen molecular H+ ion whose Schrödinger equations are unsolvable ( this p.4-lower ), quantum mechanics must artificially choose fake trial wavefunctions or basis sets.
In H2 molecule (or H+ molecule ), two hydrogen atomic 1s wavefunctions (= H-atom-A and B ) are used in the chosen trial wavefunctions ( this p.4, this p.6-7, this p.3 ).
As shown in the upper figure, in H2 molecule, even when the electron-1 approaches the other H atom-B's positive nucleus decreasing Coulomb potential energy, the electron-1's kinetic energy keeps decreasing due to the use of the H-atom-A's 1s wavefunction.
This is clearly violation of total energy conservation law (= both kinetic and Coulomb potential energies decrease ! ), so quantum mechanical molecules are false and unphysical.
So decrease in the kinetic energy is enhanced in exchange energy where the lower kinetic energy region of H atom-A (or H atom-B ) is expanded by larger probability amplitude of the other H atom-B (or H atom-A ) wavefunction near the other H atomic nucleus.
This is the trick of how the unphysical quantum mechanical exchange energy decreases the total energy (= by decreasing kinetic energy and violating energy conservation law ) to form molecular attractive bonds.
(Fig.5) The single electron-1 (or electron-2 ) must unrealistically exist in both H atom-A and H atom-B simultaneously to generate Pauli repulsive exchange energy.
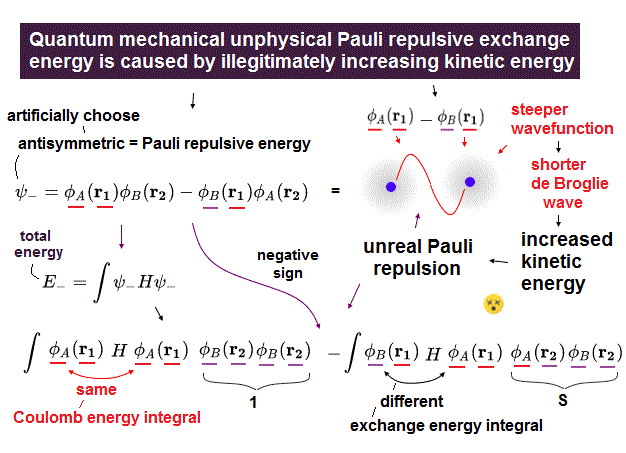
When choosing antisymmetric wavefunction where the signs of two terms of exchanging two H atom A and B wavefunctions are different (= spin is symmetric or triplet like up-up or down-down ), we can obtain Pauli repulsive exchange energy or antibonding.
↑ But electron spin-spin magnetic energy is too weak to cause strong Pauli repulsive exchange energy, so electron spin is unreal, irrelevant ( this-p.6-2.4 ).
The sign of this antisymmetric wavefunction's exchange energy integral is negative = opposite to the molecular bond attractive exchange energy integral.
So in this (negative) Pauli repulsive exchange energy integral with the opposite sign of molecular attractive bond exchange energy caused by decreased kinetic energy, electron kinetic energy increases (= without decreasing Coulomb potential energy, so total energy is Not conserved in this, either, this-p.1-2. ).
This-p.9-Fig.1.2 and p.10-1.5 say
"The effective repulsion due to Pauli exclusion stems from the change in the curvature of the
wavefunction due to the requirement for antisymmetrization in fermion systems. Higher wavefunction curvature (= shorter de Broglie wavelength )
leads to a higher kinetic energy"
"Pauli
exclusion does Not produce a force in the traditional sense"
"We call it an exchange force but it is Not really a force at all - No physical agency is pushing on the particles" ← unreal
↑ Quantum mechanical Pauli exclusion repulsion caused by (pseudo-)kinetic energy increase (= unreal exchange energy ) is Not a real force, which fact disproves quantum mechanics.
Textbooks often use the wrong explanation "antisymmetric (= antibond ) wavefunctions (= destructively-interfering wavefunctions ) decrease the electrons' probability between atoms, which may increase Coulomb electric potential energy and cause Pauli repulsion", which ignores kinetic energy change ( this-p.2-upper ).
↑ This textbooks' explanation is wrong, because the total energy is the sum of Coulomb electric potential energy + electrons' kinetic energy.
So even when the electric potential energy increases, the total energy is unchanged if the kinetic energy also decreases to conserve the total energy. ← This does Not happen in the contradictory quantum mechanical Pauli principle exchange energy.
↑ Actually, the positions of the lowest Coulomb electric potential energy is near each atomic nucleus, rather than the area between two atoms, contrary to the textbooks' explanation.
As a result, the quantum mechanical unphysical Pauli repulsive energy is caused by the drastically-increased kinetic energy compared to the Coulomb electric potential energy change
This increase in electron's kinetic energy is the driving force of unphysical quantum mechanical Pauli repulsion ( this p.9-10, this p.6-4th-paragraph, this-introduction-3rd-paragraph ).
This p.3-left-middle says
"Pauli repulsion originates in the kinetic energy increase"
↑ Pauli antisymmetric wavefunctions have steeper slope that increases kinetic energy by shorter de Broglie wavelength ( this or this p.13-2nd-paragraph ).
This-p.7-4th-paragraph says
"The opposite holds for the antibonding state,... because of its large 0.316 Eh kinetic energy, despite an attractive −0.106 Eh
potential energy contribution (both relative to H + H+). The large kinetic energy is a consequence of
the node in the wave function,.. a region of large gradients"
There is No real repulsive force or force carrier in this quantum mechanical Pauli repulsive exchange energy ( this-p.9-upper, this-p.5-upper ) caused by increased kinetic energy.
This-(4) says -- No exchange force
"The exchange interaction is sometimes called the exchange energy or exchange force. However, it is Not a true energy or force."
This-p.2-(c) says -- Unreal exchange force
"Anti-symmetrizing a wavefunction actually pushes the particles apart, while symmetrization pulls them closer together. This effect actually behaves like an effective force / pseudo-force, and is sometimes called the exchange force (= unreal pseudo-force )"
If the real repulsion is exerted between two atoms, electrons must be decelerated or slow down (= decrease kinetic energy ) by repulsion from the other atom, instead of increasing kinetic energy.
So quantum mechanical Pauli repulsive exchange energy allegedly caused by the increased kinetic energy disagrees with physical principle, so unreal.
Quantum mechanical Pauli antisymmetric wavefunctions are wrong, cannot conserve total energy nor satisfy Schrodinger equations
(Fig.6) In normal Coulomb energy integral, only Coulomb repulsion between H atom-A's electron and H atom-B's electron exists, but in abnormal exchange energy, unphysical repulsion between electrons existing in the same H atom-A (or H atom-B ) appears, which is self-contradictory.
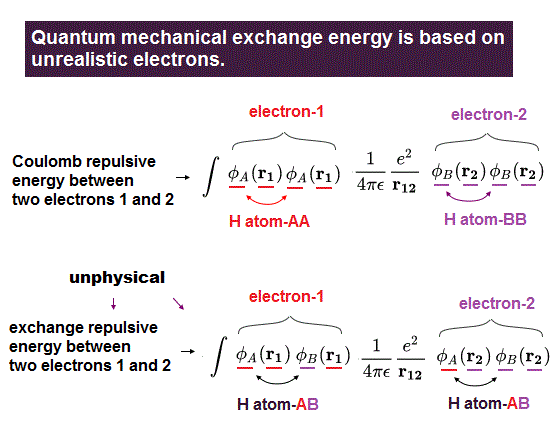
When integrating the (anti-)symmetric wavefunctions with H2 molecular Hamitonian energy, we can obtain the normal Coulomb electric repulsive energy between the electron-1 existing in H atom-A (or H atom-B ) and the electron-2 existing in H atom-B (or H atom-A ).
Coulomb energy: ∫φA(r1)φA(r1) * φB(r2)φB(r2) or ∫φA(r2)φA(r2) * φB(r1)φB(r1)
↑ In the normal Coulomb energy integral, when the electron-1 exists in H-atom-A's wavefunction, the other electron-2 must exist in H-atom-B's wavefunction.
But in the abnormal exchange energy integral,
Exchange energy: ∫φA(r1)φB(r1) * φA(r2)φB(r2)
↑ As seen here, in the abnormal exchange energy integral, even when the electron-1 exists in H atom-A's wavefuntion, the electron-2 can exist in the same H atom-A's wavefunction, which contradicts the normal Coulomb electric energy.
This is clearly self-contradiction, and shows quantum mechanical exchange energy is Not a real thing.
(Fig.7) Electrons in H2 molecules cannot conserve the same total energy E in different positions in unreal quantum mechanical Pauli principle.
In the upper Fig.7, Two hydrogen atoms of H-atom-A and H-atom-B form a H2 molecule by covalent bond, and H-atom-B and other H-atom-C repel each other by Pauli repulsion.
The total and kinetic energy of the same electron in this H2 molecule must become lower between H-atoms-A and B (= causing fictitious attractive bond energy ), and become higher between H-atoms-B and C (= causing unreal Pauli repulsion ).
Each electron feels the same Coulomb energy in positions between H-atoms-A and B and positions between H-atoms-B and C.
So the total energy = Coulomb energy (= same ) + kinetic energy (= lower or higher in different positions ) is Not conserved nor constant.
It means Schrodinger equations of quantum mechanical molecules are unsolvable (= cannot conserve the constant total energy E in different positions ), cannot predict any molecular energy.
(Fig.8) ↓ Pauli antisymmetric wavefunction is wrong, cannot satisfy Schrödinger equation.
We can prove quantum mechanical Pauli (anti)symmetric wavefunctions cannot become true solutions of Schrödinger equations, so wrong.
As shown in the upper Fig.8, quantum mechanical Pauli (anti-)symmetric wavefunctions cannot satisfy Schrödinger equation (= left-side equation contains an electron-1 in atom-A wavefunction, and right-side equation contains an electron-1 in atom-B wavefunction, which are different equations ).
It means quantum mechanics cannot solve Schrödinger equation nor predict molecular energy.

Feel free to link to this site.