
Top page ( correct Bohr model )
Back to criticizing top journals.
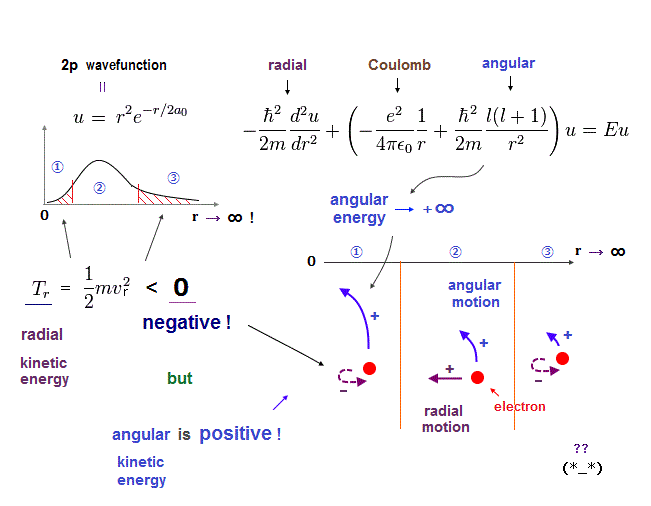
(Sh-1) Schrodinger hydrogen 2p wavefunction, negative kinetic energy parts. 

Schrodinger's "2p" wavefunctions contain radial and angular (= tangential ) kinetic energies.
As shown in this, this (3),
angular kinetic energy diverges to infinity as the inverse square of r ( 1/r2 → ∞, r → 0 ).
To cancel this increasing angular kinetic energy, the radial kinetic energy must be negative ( 1/2mv2 < 0, see ① ). So, radial kinetic energy is negative (= unreal ), but angular kinetic energy is positive !
Because the angular momentum must be always conserved (= always positive ).
Schrodinger's hydrogen contains two unrealistic parts on its both sides ( see this p.2, this p.3, this Fig.1 ).
Of course, in this real world, separating total kinetic energy into positive (= angular ) and negative (= radial ) is impossible. So Schrodinger's wave functions lack reality from the beginning.
In multi-electron atoms, wavefunctions cannot satisfy energy conservation. So unreal and incorrect.
This means asking wavefunction itself is meaningless, like NYTimes and Sean.

2015/4/18 updated. Feel free to link to this site.