

(M-1) Quantum mechanics is useless in proteins,  ↓
↓
Despite an incredible amount of research money and time wasted for the so-called "science" across the world for an extremely long time, it's still impossible to find 'miracle drugs' for curing cancers and viruses including COVID-19. ← Something wrong in the current mainstream "science" ?
Our "science" still has to rely on very old technique = vaccines which allegedly stimulate our body's unpredictable natural immune cells to produce uncertain antibodies, though severe cases of vaccinated people are increasing.
This is because the present only atomic theory = quantum mechanics and meaningless quantum field theory based on fantasy parallel worlds are unable to apply their pseudo-atomic model to practical fields such as medicine or drug discovery ( this p.1 left~right-upper ).
The only calculation tool of the old-fashioned quantum mechanics is Schrödinger equation which is completely useless, because it cannot solve or handle any multi-electron atoms, molecules, proteins.
Physicists just artificially choose fake approximate solution out of infinite choices for unsolvable Schrödinger equations which useless quantum mechanical calculation is a kind of "art" Not science.
Even this quantum mechanical approximate method of just choosing fake solutions (= without solving ) is unfeasible taking an incredible amount of time and too complicated to deal with large molecules or proteins ( this p.3, this p.11 ).
So the present method of seemingly calculating protein behavior (= still impractical, though ) relies on pseudo-classical mechanical model called molecular dynamics or molecular mechanics (= abbreviated as MM ) based on empirical pseudo-potential energies called "force field".
The problem is this widely-used pseudo-classical methods called molecular dynamics or mechanics artificially replacing actual molecular bonds by "fictional spring-and-ball models" do Not use real electrons.
So the pseudo-classical molecular dynamics or mechanics force field model lacking real electrons cannot describe any chemical or biological reactions such as bond breaking or bond forming involving electron's transfer ( this p.2 last ), hence, completely useless for claifying protein-enzymatic reactions or drug development.
The recent paper ( this introduction p.1 left last ) says the present dire situation of computational physics,
"empirical force fields (of widely-used pseudo-classical molecular dynamics or mechanics ) are still far from
perfect and, in some cases, are poorly predictive."
Scientists try to mix useless time-consuming quantum mechanics and pseudo-classical molecular mechanics lacking real electrons in vain.
It never succeeded.
The latest research paper ( this p.2 right-upper ) disappointedly admits; "such simulations of a human brain for even 1 h will not be possible until much later and may never be possible. Even if quantum computing could be adapted for molecular dynamics calculations, an enormous speed-up would be needed in order for such simulations to be performed in a reasonable time."
Useless quantum mechanical atomic theory urged physicists to fabricate "fictional future science technology" = fantasy parallel-world quantum computer which is impractical and unrealized forever.
The mainstream media is filled with "fake science news" exaggerating imaginary future fishy technology of this "pie-in-the-sky" quantum computer, using the "misleading future words" such as "will (= which means fantasy quantum computer is still useless now )", which is a kind of intellectual Ponzi scheme.
Recently, the eye-catching news claimed "Google AI called DeepMind AlphaFold could predict some protein folding by solving biggest mystery !" ← What does it mean ?
But as long as the ancient useless quantum mechanics is used in the current atomic theory, even this fashionable AI or machine learning neither changes the current dire science situation nor elucidates the underlying mechanism of how atomic interaction actually affects each protein folding.
Instead of recklessly trying to calculate protein structures based on the current useless quantum mechanical theory, this latest AI program tries to predict some protein structure based on the already-existing protein structure data (= PDB ) obtained experimentally Not by quantum mechanics.
↑ But the existing protein structure data tells us only about some static protein structure information Not about how proteins or atoms interact with each other.
So even this latest fashionable AI, which cannot utilize real atomic model or interactions due to impractical quantum mechanics, is still unable to explain protein-protein interactions or biological reactions required for developing effective drugs.
The 2nd-last paragraph of this news (= Unlocking new possibilities section ) just vaguely says "there are still many questions to answer. Not every structure we predict will be perfect. There’s still much to learn, including how multiple proteins form complexes, how they interact with DNA, RNA, or small molecules.."
↑ So this latest AI allegedly predicting some protein structure based on the past protein database still cannot explain or predict any molecular or protein interactions, hence, the fashionable AI or machine-learning, which sounds good, will be useless for predicting biological reactions or drug discovery forever, as long as the ancient quantum mechanics is blindly applied to the basic atomic theory.
The most serious problem is No present quantum mechanical atomic models can deal with real Coulomb forces for pushing atoms or changing molecular bonds in biological reactions, instead, the impractical quantum mechanics and pseudo-classical molecular dynamics try to find illusory pseudo-potential energies lacking real force concept or force carriers in vain.
Hence, such an unrealistic quantum mechanics lacking real mechanical forces cannot be utilized for clarifying any biological or chemical reactions involving movements of electrons atoms and molecules.
→ Designing and constructing useful molecular devices for curing diseases by moving or putting together smaller atoms or molecules are impossible as long as such an unrealistic quantum mechanical model lacking real forces is used to explain (pseudo-)atomic interactions.
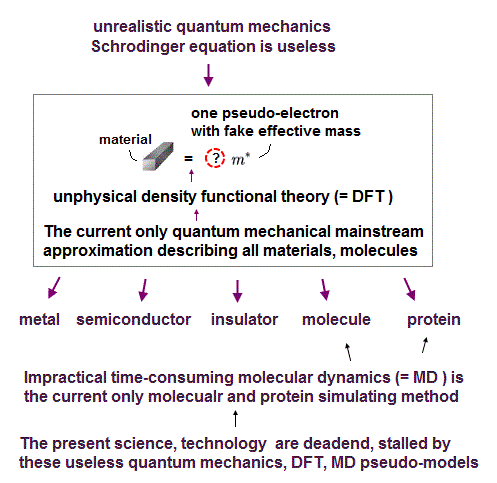
Instead of the useless time-consuming original quantum mechanical methods, its approximate version called density functional theory (= DFT ) is the most widely used for seemingly describing some protein behaviors including the recent viruses ( this p.6 right middle ).
The problem is this most widely-used quantum mechanical fishy approximation DFT or Kohn-Sham theory is far from reality.
Because DFT outrageously replaces the whole many-electron materials, molecules, proteins by one fake electron model ( this p.2, this p.6 ) which unscientific manipulation requires physicists to make up effective pseudo-potential energies called "exchange-correlation functionals" which pseudo-potential energy's exact form is unknown ( this p.1, this p.27 ).
Physicists have to artificially choose arbitrary pseudo-potential energies called "exchange-correlation functionals" in different situations or materials, hence, DFT has No ability to predict new atomic behavior (= DFT is one of artificial semi-empirical methods, Not first-principle as seen in this p.23 ).
There is No universally exact pseudo-potential energy or exchange-correlation functional in any quantum mechanical pseudo-models including the most-widely used DFT approximation.
But all the present physicists across the world blindly believe such an unrealistic quantum mechanics or its most popular approximation DFT as the only atomic theory ( giving up clarifying the real underlying physical mechanism ), trying to find illusory universal pseudo-potential energy or exchange-correlation functional in vain ( this p.2 ).
No functional (= DFT pseudo-potential energy ) is accurate for all properties of interest. No matter what functional is ‘invented’ someone will always find a case where it fails ( this p.17 )
No matter what forms of pseudo-potential energies or functionals are chosen, DFT is unable to explain metals, oxygen, water molecules, van der Waals intermolecular interactions, any chemical reactions involving electron's excited states or charge transfer ( this p.1 right, this p.31-32 )
This site (= p.1 ) mentions such a failed DFT method,
"the
exact (exchange-correlation) functional E[ρ] is not known and, some believe, is
so complicated that it is practically unknowable.."
"..As of 2011, there are hundreds of density-functional approximations to choose from. Most of them perform remarkably well for certain types of problems and fail for others ( this p.20 ).."
".. one should always keep in mind that practical computational chemistry never deals with the exact density functional, but only with density functional approximations. If we knew the exact functional, then every DFT calculation would be exact. When we say “DFT fails, we mean that the density functional approximation we chose to use fails to give the correct prediction."
Humans already have excellent and useful technology for observing and manipulating each single atom and molecule one by one using atomic force or scanning probe microscopy.
So using this already-existing excellent technology of watching and manipulating each single atom (= of course, pushing or pulling each atom needs "real forces" ), we should construct any sophisticated practical molecular nano-machines to eradicate harmful viruses or cure any deadly diseases such as cancer. ← But we still cannot do this due to the unrealistic quantum mechanical basic atomic theory.
Using the current atomic force microspoce technology → manipulating and measuring forces of each atom and small molecule → find the "common physical principle" or real atomic model which can describe the measured atomic forces or sources of such forces. → applying such measured and realistic atomic models to small molecules first by gradually confirming the validity of the theory or model in small molecules using atomic force microscopes.
→ By putting together the measured and confirmed small molecules or atoms (= after these molecular behaviors and atomic forces are confirmed to be explained by real atomic model using atomic force microscopes ), we can design and build any useful bigger molecular nano-devices in the same way as we design and build any houses, cars and machines combining small parts (= or small molecules ) with already-known shapes and functions.
↑ This practical way of utilizing the current excellent atomic force microscopy technology for building useful molecular devices by combining small molecules is impossible as long as we rely on unrealistic quantum mechanical atomic model to describe small basic atomic or molecular interactions.
All the current physicists try to do contradictory things = blindly relying on unphysical quantum mechanical one-pseudo-electron approximation = density functional theory (= DFT ) which cannot model real atomic forces between separate different atoms, in order to explain the measured atomic forces by microscopes ( this p.2 left, this p.2-right-lower, this p.9-middle ).
Measure actual forces by microscopes
→ But quantum mechanical model cannot describe these measured forces
→ Instead, physicists try to pursue or create illusory universal pseudo-potential energies or functionals in one-pseudo-electron approximation DFT in vain.
As I said, there is No universal exact pseudo-potential energy or exchange-correlation functional in this DFT model, hence, each time physicists deal with different atoms or molecules, the existing pseudo-potential energy models turn out to be useless, disagreeing with newly observed molecules, and eventually they have to look for or create new other artificial pseudo-potential energies or illusory universal functionals, forever.
↑ The present computational physics or quantum chemistry falls into a meaningless infinite loop, which is why we cannot use quantum mechanical model for practical fields such as drug development.
As long as physicists blindly use the present unrealistic quantum mechanical DFT atomic model (= outrageously replacing actual many-electron molecules by one-pseudo-electron model ), they can never move forward to design or construct bigger useful molecular nano-devices by putting together small molecules or atoms, because quantum mechanical model lacking physical forces cannot tell you in which direction each atom or molecule will move even when external forces are applied to it.
This is the main reason why we still have to rely on very old technique = unpredictable vaccine without knowing precise microscopic molecular mechanism of how our body's natural whimsical immune systems respond to vaccines, what kind of side effect will occur is uncertain.
The useless quantum mechanics originates from the fact that its unphysical probability wavefunction or electron cloud model can Not exert real Coulomb forces in generating molecular bonds or interactions between atoms.
The quantum mechanical electron cloud must always be thinly spread and distributed symmetrically around the nucleus, hence, quantum mechanical static electron cloud cannot behave like real moving particles.
↑ The thinly-spread quantum mechanical electron clouds are unable to move to the left or right side of the nucleus to approach the other positively-charged nucleus or avoid the other negatively-charged electron.
The quantum mechanical spread electron clouds are unable to form realistic molecular bonds by using strong Coulomb attractions between negative electrons and positive nuclei of two neutral atoms. ← Real Coulomb attraction cannot be used in quantum mechanical pseudo-molecular bonds.
As a result, quantum mechanics, which cannot utilize real Coulomb electric force for molecular interactions between two neutral atoms, has to rely on the contradictory and "unrealistic pseudo-energies called exchange energies" lacking real physical (exchange) forces for causing molecular bond attractive energies or Pauli repulsive energies between atoms by putting every electron in all different distant atoms simultaneously and violating energy conservation law.
All intermolecular forces consist of weak attraction called van der Waals force and strong repulsion based on Pauli exclusion principle, which mixed intermoleular forces cannot be explained by quantum mechanics or its pseudo-exchange interactions which can only handle one of attractive or repulsive exchange energies (= Not both or mixed forces simultaneously ).
Quantum mechanics unreasonably claims all these intermolecular or interatomic energies have to be described by "unphysical antisymmetric wavefunctions" where every single electron must always exist in all different (separate) atoms or orbitals simultaneously.
Outrageous antisymmetric wavefunction rule: = exchanging any two electrons existing in different separate atoms or orbitals does Not change the form of the whole wavefunction ( except for the sign flip, this p.11-12 ).
↑ This ridiculous draconian antisymmetric rule (= has nothing to do with the real physical world ) demands every single electron should always exist in all different (separate) atoms or molecules simultaneously like fantasy quantum mechanical superpositon or parallel worlds.
Of course, each single electron is indivisible. ← Such an indivisible single electron must always exist in all different distant atoms or orbitals. ← All different distant atoms or molecules also become unrealistically indivisible and inseparable ! ← Quantum mechanical unrealistic exchange energy rule lacks real physical forces between separate atoms.
Due to this inseparable electron unrealistically existing in all different distant atoms simultaneously, the pseudo-potential or exchange energies cannot have real "exchange forces" or force carriers ( this p.11 ). ← What causes these molecular exchange attraction or Pauli repulsion cannot be defined or expressed by real objects according to the stupid quantum mechanics !
But this unrealistic exchange energies are supposed to be a main generator of any quantum mechanical molecular bonds instead of useless quantum mechanical Coulomb energies ( this p.3-4 ).
Weak intermolecular attractive force = van der Waals force is known to be caused by Coulomb attraction between negatively-charged electrons and positively-charged nuclei belonging to two adjacent neutral atoms whose electrons temporarily move to the left or right side of each nucleus (= causing electric dipole ), approaching the other nuclei and avoiding the other electrons.
But the quantum mechanical unphysical electron cloud cannot move or be localized to the left or right side of the nucleus, hence, the quantum mechanical electrons intrinsically have No ability to describe any Coulomb attractions including strong molecular covalent bonds and weak intermolecular van der Waals attraction.
As a result, the quantum mechanics and its pseudo-approximation DFT model cannot explain van der Waals attractive energies using realistic Coulomb attraction.
Hence, it has to artificially create and add other new ad-hoc pseudo-potential energies or exchange-correlation functionals to seemingly explain the van der Waals attraction (= add new ad-hoc intermolecular van der Waals pseudo-potential energy or functional to the original molecular covalent bond's functionals instead of explaining van der Waals attraction using real Coulomb attraction between moving electrons and nuclei, this p.5-6 ).
These artificially-added van der Waals pseudo-potential energies (= Not based on real Coulomb force ) or functionals often failed to explain various actual intermolecular energies ( this p.28, this p.1 ).
The original Coulomb force can be expressed by very simple relation.
But depending on different positional relationship between different electrons and nuclei, infinite kinds of different forms of complex Coulomb potential energies are generated, one of those complex Coulomb attractions is intermolecular van der Waals attraction.
↑ One-pseudo-electron approximation DFT has to do the impossible thing = recklessly trying to find illusory universal pseudo-potential energy or exchange functional out of infinite kinds of different pseudo-potential energies or functionals prepared to deal with different situations or different combinations of molecules involving different kinds of complex Coulomb energies caused by different positional relationships between electrons and nuclei.
↑ This is impossible. Thre is No such thing as the dreamlike universal pseudo-potential energy or functional describing any kinds of different complex Coulomb or van der Waals potential energies, though all the present physicists are desperately trying to find those illusory universal DFT pseudo-potential energy or functional in vain. ← now
This current miserable "science" or academic state is caused by the fact that the quantum mechanical electron cloud always spreading symmetrically around the nucleus is intrinsically unable to deal with real Coulomb forces even between two simple atoms.
All molecular or intermolecular attractions such as covalent bonds and van der Waals forces must be originally explained by "Coulomb electric attraction" between positive nuclei and negative electrons by moving and adjusting electron particles' position around the nucleus.
↑ This adjustment of strength of Coulomb attraction by moving and changing the positions of electron particles around the nucleus is impossible in unphysical quantum mechanical wavefunction or electron cloud which must always spread symmetrically around the nucleus to keep its electron cloud's kinetic energy from increasing unrealistically.
In fact, quantum mechanical unphysical wavefunction or its electron cloud (= it's like each electron particle is divided into infinite number of infinitesimal charges and spread symmetrically around the nucleus ) is also unable to explain why F-F (= fluorine ) molecular bond energy becomes smaller than Cl-Cl (= chlorine ) bond, though Cl-Cl bond length is longer than F-F bond length.
↑ Originally, longer Cl-Cl bond energy should be smaller than shorter or stronger F-F bond, but actually, the opposite is true (= shorter F-F bond energy is smaller than longer Cl-Cl bond ), which stronger Coulomb repulsion between electrons in F-F bond can be explained only by real atomic model with point-like electron particles Not by quantum mechanical thinly-spreading electron clouds.
↑ Coulomb repulsions between atoms with a small or large numbers of valence electrons play a crucial role in deciding hydrophobic or hydrophilic properties between neutral atoms or molecules, which cannot be described by unphysical quantum mechanical electron clouds without artificially creating or adding ad-hoc pseudo-potential energies
Ordinary electric current is also explained only by realistic atomic model with moving electron particles, Not by unphysical static quantum mechanical electron cloud.
In the quantum mechanical wavefunction, even a single electron must always spread symmetrically around the nucleus as a vague electron cloud. → Quantum mechanical electron cannot move to the left or right side of the nucleus due to its stupid "probability" rule.
→ Quantum mechanics cannot use real Coulomb forces in any molecular bonds, intermolecular van der Waals attractions and Coulomb repulsions between atomic electrons.
→ Each time physicists deal with different atoms or molecules, they have to artificially create new ad-hoc pseudo-potential energies in vain. ← Dreamlike universal pseudo-potential energy or exchange functional will never be found in DFT which useless pseudo-approximate method is the most widely used now.
→ Designing and constructing useful molecular nano-devices by clarifying molecular or protein interactions are impossible as long as we rely on unrealistic and contradictory quantum mechanical model which cannot utilize real physical forces such as Coulomb force and Pauli repulsion.
→ Very old vaccine technique blindly believing unpredictable natural immune responses is the only choice for tackling varuses forever. → Unreasonable vaccine mandates restricting freedom and free thoughts or science are rampant. ← now.
It's impossible to describe actual protein's movement or interaction using the current quantum mechanical impractical methods, its stupid one-electron approximation DFT or pseudo-classical molecular dynamics lacking real electrons or Coulomb forces.
Protein conformational change mainly caused by their amino acids' rotations must be treated like a "rigid body rotation", and proten-protein interaction must be explained by real physical forces between proteins. ← Even this simple manipulation is impossible in the current most-widely used DFT approximation where all different atoms or electrons are treated like a fictional inseparable single pseudo-electron.
For example, in the protein's amino acids of this figure lower, when some external force is applied on the oxygen atom (= O1 ), this appplied force is transmitted immediately through the whole amino-acid molecule (= consisting of multiple atoms tightly bound to each other by covalent bonds ) and tries to rotate the whole amino-acid (= yellow plane rotates like a rigid body consisting of multiple tightly-bound atoms ), and the distant H atom of the same amino acide also rotates and collides with the other O2 atom.
↑ This kind of actual protein's amino acid motion cannot be explained by the current quantum mechnaical methods or pseudo-classical molecular mechanics.
Because all these current impractical methods must artificially create ad-hoc pseudo-potential energies first, instead of considering real physical forces or force carriers (= infinite kinds of complex pseudo-potential enregies are needed to deal with infinite kinds of different situations or positional relationships between different atoms ) to describe this rigid-body like amino acid rotation.
↑ Quantum mechanical method = find and create pseudo-potential energies first ( instead of considering real Coulomb forces first ) → The derivative of those pseudo-potential energies means fictitious forces, which are Not actual forces. ← As I said, the dreamlike universal pseudo-potential energy is impossible to find.
In order to explain actual proteins' behavior, we have to treat different molecules as "separate real oblects with real electrons moving around nuclei" which can exert real Coulomb forces on other molecules.
↑ If we can know the directions of "forces" ( instead of creating pseudo-potential ) from the beginning, we can easily predict in which direction each molecule or protein will move in biological or chemical reactions. → Applicable to various practical fields such as medicine.
In unreal quantum mechanical model lacking real Coulomb or Pauli force and pseudo-classical molecular force field model lacking real electrons, physicists often allocate fictional atomic charges to each whole neutral atoms, because all molecular interactions should be originally explained by combining the most important Coulomb electric forces between electron or nuclear negative or positive charges.
↑ But this quantum mechanical or pseudo-classical methods of artificially replacing the "original neutral atoms" by "fictional charged atoms" are contradictory and unable to explain actual molecular behaviors.
For example, they often give pseudo-positive charge to neutral hydrogen atom (= H ), and pseudo-negative charge to neutral oxygen atom (= O ) to explain intermolecular attraction or hydrogen bonds in water molecules.
↑ But these fictional atomic charges temporarily given to the whole neutral atoms are Not real charges, because they are unobservable ( this p.27, this p.5 ).
Actually, if neutral hydrogen atom is treated like a pseudo-positively-charged atom, two pseudo-positive hydrogen atoms must always repel each other, hence, cannot approach each other, which contradicts the actually-observed van der Waals attraction between two neutral hyrogen atoms.
"Neutral atoms" should be treated as "neutral atoms" as they are, without relying on fake atomic models of pseudo-charged atoms !
Coulomb or van der Waals attractions between any neutral atoms must be explained by real atomic model with real moving electrons Not by quantum mechanical static electron cloud where electron clouds can neither move to the left or right sides of the nucleus nor cause interatomic Coulomb attraction between neutral atoms without relying on unphysical exchange or pseudo-potential energies .
In the realistic atomic model, strong molecular covalent bonds are naturally caused by Coulomb attraction between positive nuclei and movable negative electrons approaching nuclei and avoiding other negative electrons.
In noncovalent intermolecular bonds, electrons belonging to different distant atoms cannot always cooperate or synchronize with other distant electrons to avoid each other, hence, electrons of two distant molecules sometimes approach each other and cause weaker Coulomb attraction or weaker van der Waals force between distant molecules than stronger covalent bond attraction between closer atoms where electrons can always synchronize and avoid each other perfectly.
Using the current excellent atomic force microscope technology ( or its improved versions, because at least two probe arms are necessary for grabing or measuring exact atomic forces. All the current atomic force microscopes have only one probe which cannot be utilized for constructing bigger molecular nano devices ), we should apply each measured Coulomb or Pauli forces to real atomic model with movable electrons, and gradually construct bigger molecular devices by putting together smaller molecules whose atomic interactions are confirmed by the microscopes one by one.
↑ This practical use of atomic model for building useful molecular devices for tackling annoying viruses or curing diseases is impossible forever in the current quantum mechanical unphysical wavefunction or electron cloud model which cannot use real forces or real separate movable electrons, because quantum mechanical wavefunction unreasonably mixes two conflicting concepts of a particle and wave.
See previous version of criticizing top journals.

2021/8/20 updated. Feel free to link to this site.