
(Fig.1) Light frequency f shows light is wave.

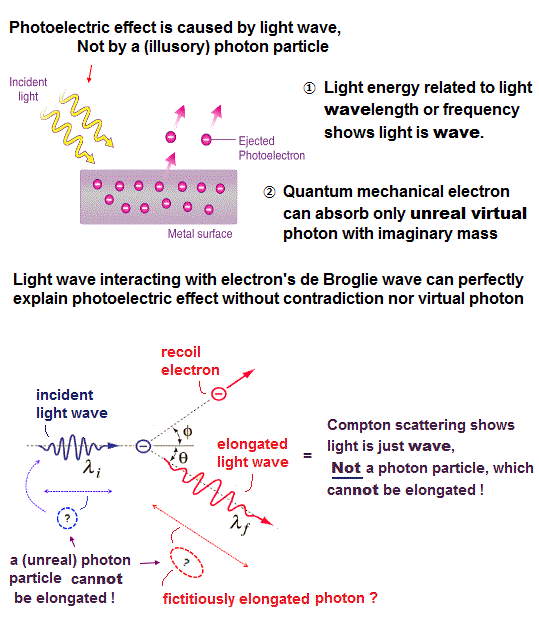
It is often said that Einstein's photoelectric effect showed that the light also has the particle nature as a photon. But this is a big lie.
In photoelectric effect, when the frequency (= f ) of the incident light is above some threshold, an electron inside metals is ejected. That's all.
NO photon is seen in photoelectric effect !
All textbooks and news mislead you into believing false logic !
(Fig.2) Mass squared of virtual photon can be negative ! → QM is wrong

In fact, the photon emitted or absorbed into electron in quantum mechanics is all "virtual", NOT real. It means quantum mechanics is unrealistic and false.
The ratios of energy to momentum in electron and photon are different, so an electron cannot emit a photon conserving both energy and momentum.
So in Feynman QED, a photon emitted from an electron is virtual, not real, because the mass squared of this virtual photon can be negative ( this p.2 below ).
Of course, the mass squared of all real particle must be positive.
So the photon in quantum field theory is completely unrealistic.
Though Einstein predicted a photon, its photon must be virtual, violating Einstein. ← Ironical outcome !
(Fig.3) Electron's de Broglie wavelength λ = h/p, p = mv is momentum
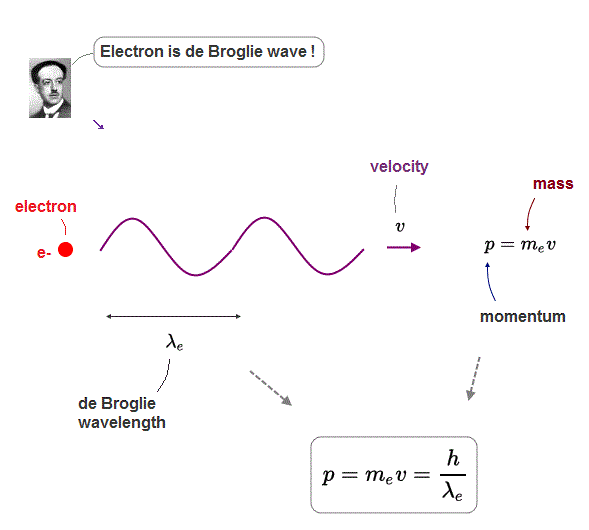
Many experiments proved that each electron has its de Broglie wavelength λ
This wavelength λ is the resiprocal of the electron's momentum p = mv ( m and v are electron's mass and velocity ).
(Fig.4) Light energy E = hf ( h is Planck constant, f is frequency )
Photoelectric effect showed that the light energy E is proportional to its frequency. E = hf where h is Planck constant.
Then, how about the electron's frequency ? The frequency of electron's de Broglie wave is also proportional to its energy ?
(Fig.5) v = electron velocity fe, λe are electron frequency and wavelength
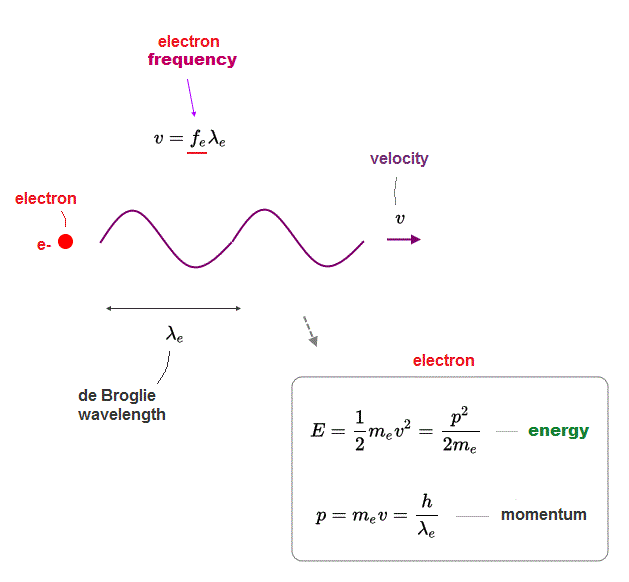
According to wave mechanics, the velocity v is equal to its frequency f times wavelength λ, where this λ means electron's de Broglie wavelength.
E is electron's ( kinetic ) energy, which is equal to 1/2mv2, where m and v are electron's velocity and mass.
(Fig.6) Wave relation + de Broglie relation = E is proportional to frequency f
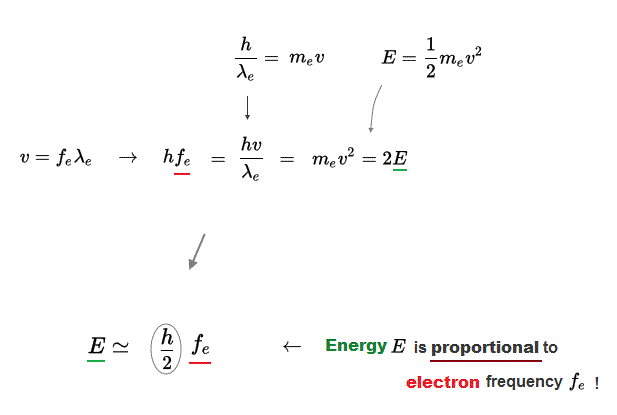
Using de Broglie relation and wave mechanics, we can prove that electron energy E is also proportional to its wave frequency fe.
Only the factor 1/2 in electron is different from the light energy.
(Fig.7) Light frequency flight is half of electron frequency fe
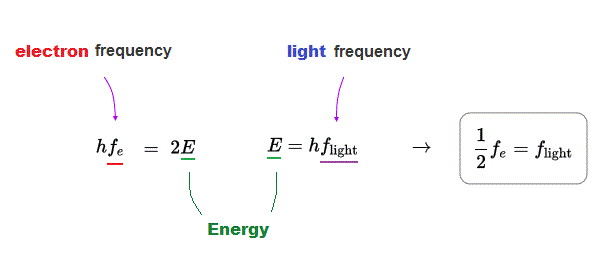
Comparing energy relations of light and electron, we find that the light frequency is half of electron de Broglie wave frequency of the initial state.
(Fig.8) ↓ An electron is losing its energy E, emitting light of frequency f
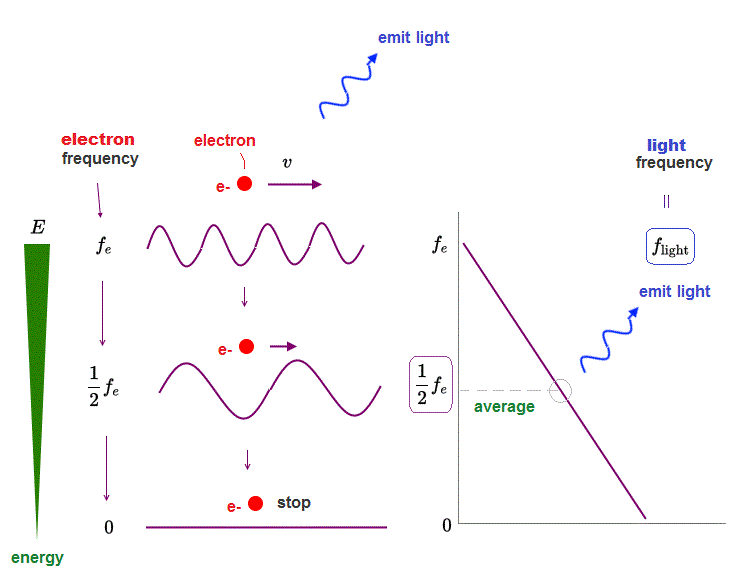
Here is an electron having kinetic energy E and it is losing the energy by emitting light. As the electron's energy decreases, its frequency fe decreases, too.
So the average frequency (= flight ) of the light emitted from the electron should be half of the initial electron's frequency (= fe ).
As a result, we can prove the frequency relation: flight = 1/2 × fe
(Fig.9) Light emitted from orbital electron from excited to ground states.
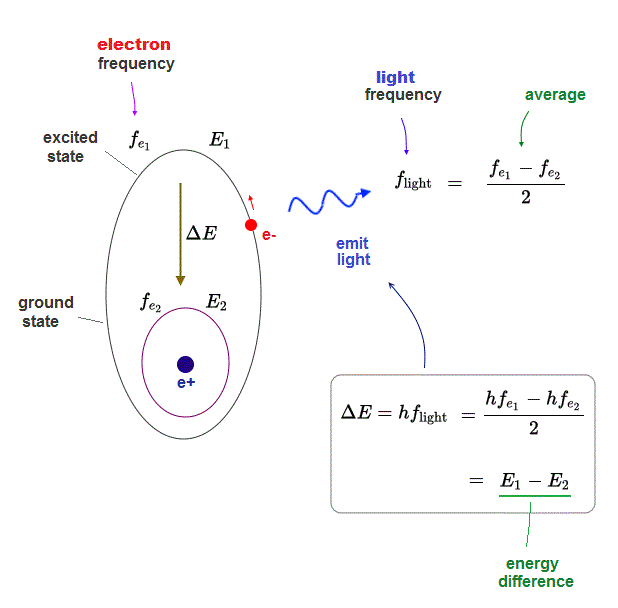
In the same way, when an excited electron backs to the ground state, the relation between the light and electron frequency is valid.
As the electron is approaching the ground state by emitting light, the gap between the initial (= fe1 ) and final (= fe2 ) frequencies is smaller.
So the average light frequency is half of the initial difference in electron's frequency.

2016/11/18 updated. Feel free to link to this site.