(Fig.1) Quantum mechanics just artificially adjusting free parameters cannot predict any atomic energies.

Quantum mechanics relies on Schrödinger equations where the conserved constant total energy E is the sum of electrons' kinetic energy expressed as differential operators acting on atomic wavefunctions and Coulomb potential energy among electrons and nuclei.
Quantum mechanical Schrödinger equation for only one-electron hydrogen atom can be solved, which solution agrees with successful Bohr atomic model ( this-p.12, this-4.133 ).
Quantum mechanical Schrödinger equations for multi-electron atoms ( such as helium ) and hydrogen molecule ion (= H+ ) are unable to have true solutions or true wavefunctions to conserve the constant total ( ground-state ) energy E (in any different electrons' positions ), so quantum mechanical Schrödinger equations and wavefunctions proved to be false ( this p.2-upper, this p.7 ).
Quantum mechanics, which is useless and unable to predict any atomic energies, just artificially chooses fake trial wavefunction or basis sets (= any forms of trial wavefunctions and any numbers of free parameters are allowed to be chosen ) for unsolvable Schrödinger equations to find the lowest atomic energy out of infinite choices of trial wavefunctions ( this-p.3-lower ), which takes infinite time, so unable to predict any atomic energies ( this-p.9-last ), which ad-hoc approximation is called variational methods ( this p.25 ).
Unsolvable Schrodinger equations have to find fake solutions giving the lowest atomic energies out of infinite choices of wavefunctions and parameters by approximate variational methods ( this-(2) ).
↑ But it is impossible to look into infinite numbers of parameters, so quantum mechanical (variational) methods are useless, unable to predict any atomic or molecular energies ( this p.2-2nd-last-paragraph ).
This-p.1-last says -- No quantum mechanical prediction
"The (quantum mechanical variational) theorem does Not tell us (= cannot predict ) how far above the ground state energy"
If this variational methods, which should give the upper bound to a true ground state lowest energy, can find wrong solutions giving energies lower than actual atomic energies, the quantum mechanics is proved wrong ( this-21.1 ).
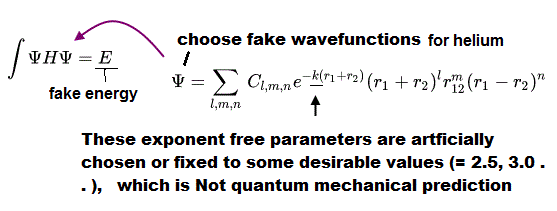
Actually, physicists can find these wrong solutions disproving quantum mechanics, so they deliberately fix some free parameters called exponent to artificially prevent their chosen fake solutions from giving energies lower than actual atomic energies.
(Fig.2) Quantum mechanics has to artificially choose free parameters to adjust calculated (fake) atomic energies, which cannot predict any true atomic energies.
In fact, in all these quantum mechanical (variational) methods for the unsolvable useless Schrodinger equations, physicists must deliberately manipulate free parameters (= exponent parameters k, ζ, ξ, η of e-kr, e-ζ r, e-ξr, e-ηr..) of artificially-chosen fake wavefunctions to seemingly obtain (fake) total energy close to experimental value ( this p.6-7 ), which is Not prediction of quantum mechanics.
This or this-p.1 says -- No exact solution
"Hamiltonian (= Schrodinger equation's atomic energy ) that cannot be solved exactly and where
perturbation theory cannot be applied"
"One possibility is to use the variational principle: it does Not give you the exact answer but gives you an upper bound to the energy" ← This is disproven here, so quantum mechanics is wrong.
↑ If the current only quantum mechanical approximate method called variational method ( this-p.1~6 ) choosing fake wavefunctions for unsolvable Schrodinger equations gives wrong energies lower than the true atomic energies (= break the upper bound ), it disproves quantum mechanics. ← This actually happened.
↑ Actually, physicists need to intentionally fix the free exponent parameter k of chosen (fake) wavefunctions to some desirable values (= so quantum mechanics cannot predict atomic energies ), because if they rigorously try to find the exponent parameter k giving the lowest energy ( out of infinite choices ), this can give wrong energies lower than true atomic ground-state (= lowest ) energies ( this p.6-IV says The exponents are considered as adjustable parameters. ), which fact disproves quantum mechanics.
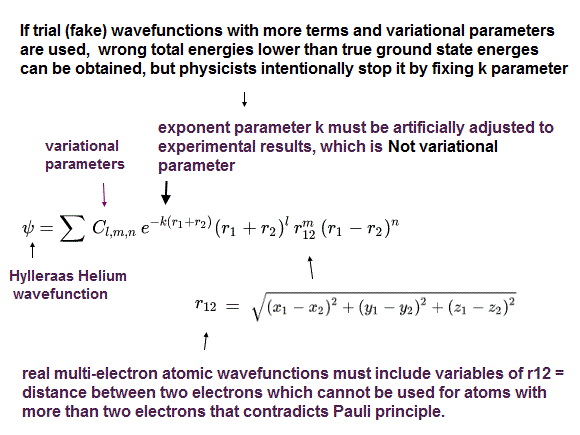
Only in a two-electron Helium (or lithium ) among the unsolable multi-electron atomic Schrodinger equations, physicists artificially choose Hylleraas-type (fake) trial wavefunctions including variables of interelectronic distance (= r12 = distance between two electrons-1 and 2, this-p.2 ), which can give (fake) total energies lower than other trial wavefunctions in variational methods.
The problem is that the calculation (= integral ) of this chosen Hylleraas trial wavefunctions is impractical, too time-consuming to apply to atoms with more than three electrons.
↑ It means quantum mechanics can Not obtain energies matching experimental energies of atoms with more than three atoms, even in its illegitimate approximate (= variational ) methods.
(Fig.2') Quantum mechanics just choosing free parameters cannot predict atomic energies.
Here we show that the current quantum mechanical (variational) methods rely on freely-adjustable parameters (= instead of finding the parameters giving the lowest possible energy ), which is useless, can Not predict (Helium) atomic energies.
This paper about the current best quantum variational method for helium ground-state energy's p.1-(1)(2), p.3-Table.I says "where αi, βi and γi are complex (free) parameters (= exponent parameters of the artificially-chosen helium Hylleraas wavefunctions ) generated in a quasi-random manner:.
↑ Just adjusting parameters by quasi-random manner giving the helium ground state energy means they can give wrong energies lower than true atomic energies, if they really rigorously try to find exponents parameters giving as low energies as possible out of infinite parameter choices, which means quantum mechanics is wrong."
This paper-p.12-2nd-paragraph says -- Artificially-fitting parameters
"Also, I know of no published theoretical attempts to explain the excellent convergence found by Korobov with his highly nonlinear fitting of the
trial wavefunction..
Korobov’s approach (= upper-quasi-random manner ) may be likened to the work of fitting the Hydrogen
radial wavefunction with a set of gaussians, using floating exponents.
This sounds plausible, but at present it is just more handwaving about flexibility."
This or this-paper (= one of today's best quantum variational methods for helium ground-state energy ) ↓
p.4-left-last-paragraph also says -- Free exponent parameters
"The orbital exponent may be treated as a nonlinear (= exponent ) variational parameter,
though this is Not strictly necessary (= they don't try to find the lowest energy by using exponents as strictly-variational parameters )"
p.5-(24), p.9-Table.V -- Wrong variational method
They intentionally fixed one non-linear parameter β = 1 and another exponent parameter α = 2.20 (= if they more strictly vary these parameters, they could find fake energies lower than true ground state energies, which disproves quantum mechanical variational method that must always give energies higher than true atomic energies ).
Schwartz intentionally fixed an exponent parameter k to 3.5 (= k is Not used as a variational parameter finding the lowest energy, this-introduction-(3), p.1147-Results says the parameter k was fixed at the artificially-chosen value of 3.5 ) to illegitimately obtain Helium ground-state energy by quantum variational method.
Also in the recent calculation, he fixed (= instead of vary ) the exponent parameter k at (the artificially-chosen value of ) 2.0, which was used as an adjustable scale parameter (= to prevent the calculated values from getting lower than true helium energy ) not as a true variational parameter giving the lowest possible energy in obtaining helium ground state energy based on Hylleraas trial wavefunctions ( this p.2-(0.3) ).
Another research also intentionally fixed exponent parameters (= η, ζ ) of helium Hylleraas wavefunctions at (artificially-chosen values of) 6.00 or 6.50 ( this-p.6-lower~p.7-Table.1 ) to deliberately prevent their calculated energies from being lower than true helium ground-state (= lowest ) energy.
Drake also treated the exponent nonlinear parameters as freely optimized scale factors ( this p.4-Table.IV ).
↑ It means quantum mechanical variational methods can get wrong energy values lower than true ground state energies (= in atoms such as helium ) if physicists use various forms of wavefunctions (= including interelectronic distance like Hylleraas ) and really try to find parameters giving the lowest possible energies.
↑ This fact of chosen wavefunctions giving wrong energies lower than true atomic energies disproves quantum mechanics or its variational method choosing (fake) trial wavefunctions that must always give energies higher than the true atomic lowest ground-state energies.
They intentionally fixed those (exponent) parameters at some round numbers (= like exponent scale parameter k = 2.0, this-p.2-(0.3) ) to avoid lowering the calculated values below the true ground state energies. ← fake variational method
This or this-p.1-introduction-4th-paragraph (and p.5-Table.1 ) says "However, according to recent experimental result (Bergeson et al., 1998), the results from the techniques reported above overestimated the helium ground-state energy" ← Quantum mechanical variational methods turned out to be Not variational, so false.
Also in lithium case, quantum mechanics just chooses artificially-fixed exponent parameters like helium ( this-p.2-right-last-paragraph ), which disproves quantum mechanics and its variational method.
Quantum mechanical Pauli antisymmetric wavefunction is wrong, cannot be applied to more than two electrons like lithium.
The lithium wavefunction (= this-(4) ) contains only one symmetric relation between electrons 1 and 2 (= attractive exchange energy ), while other relations between electrons 2 and 3, and electrons 1 and 3 are antisymmetric (= Pauli repulsive exchange energy ).
↑ This is wrong, because if the relation between two electrons with spin up (= α ) and down (= β ) is symmetric in three electrons with up, up, down spins, not only relation between electrons 1 (= up spin ) and 2 (= down spin ), but also relation between 2 (= down spin ) and 3 (= up spin ) must be symmetric.
(Fig.3) Quantum mechanical unsolvable Schrodinger equations have to artificially choose fake unphysical trial wavefunctions in the impractical CI.
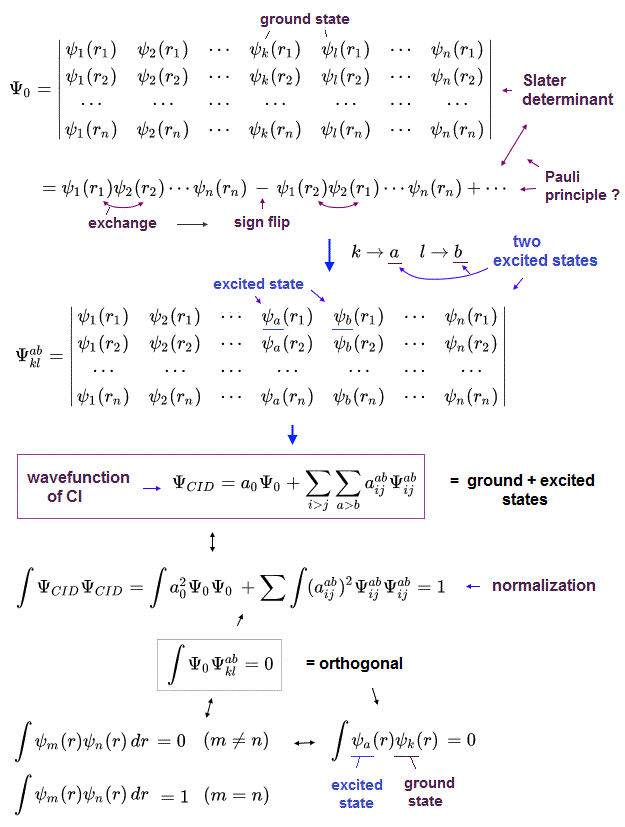
Quantum mechanical ( artificially-chosen ) wavefunctions (for unsolvable useless Schrodinger equation ) obeying the ad-hoc Pauli principle must be expressed as (unphysical) antisymmetric wavefunctions or Slater determinants, whose Schrodinger energy approximate calculation is called Hartree-Fock (= HF ) equations ( this p.6 ).
These quantum mechanical approximate Hartree-Fock (= HF ) or molecular orbital methods are known to be unable to give the total energies close to experimental values even in their chosen trial wavefunctions. ← the difference between this Hartree-Fock equation and true energy is called correlation energy.
The current most-widely used quantum mechanical approximate methods to seemingly get this correlation energy beyond Hartree-Fock is one-pseudo-electron density functional theory (= DFT ) that just artificially chooses fictitious exchange-correlation energy functional and pseudo-potential ( this-p.3-5 ), so DFT is fake ab-initio unable to predict any values.
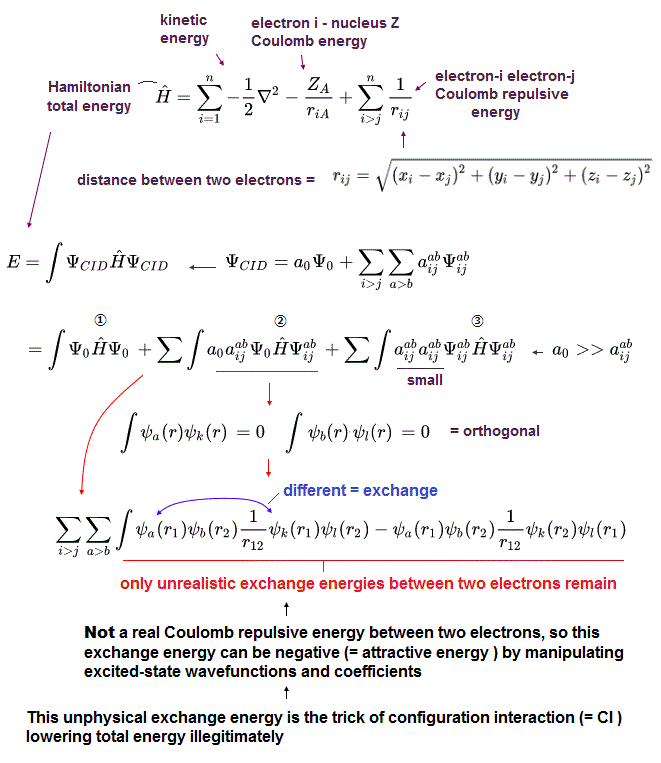
The unsolvable useless quantum mechanical Schrödinger equations have to rely on the impractical time-consuming approximation called configuration interaction (= CI, this-p.5-last ).
In this impractical (full) CI, the artificially-chosen trial (fake) wavefunctions (= ΨCID ) are expressed as the linear combination of more than one (unphysical) antisymmetric Slater determinants ( this-p.3-4, this-p.21 ).
This impractical CI includes the fictional excited atomic wavefunctions ( this-p.8, this-p.8 ).
They artificially choose and adjust these fictitious exchange and ground-sate atomic trial wavefunctions to give lower (fake) total energies.
↑ The main components of this CI (illegitimately) lowering total energy is the (unreal) exchange energies between (artificially-excited) electrons ( this-p.30-37, this-6.5.4~ ).
Because the artificially-chosen excited and ground-state trial wavefunctions are often orthonormal to each other (= integral of these wavefunctions are artificially made to be zero ), which cannot make the exchange energy between two (fictitious) electrons zero ( this-p.1,7, this-p.3 ). which remain as the (fictitious) correlation energy.
↑ Even this impractically time-consuming full CI cannot give (fake) total energies matching experimental values, because multi-electron atoms with more than three electrons cannot use Hylleraas wavefunctions that can give wrong energies lower than the true atomic energies, which disproves quantum mechanics.
(Fig.4) Coupled cluster (= CC ), which is Not variational, can give wrong energies lower than true ground state energy, so CC is Not a right theory.
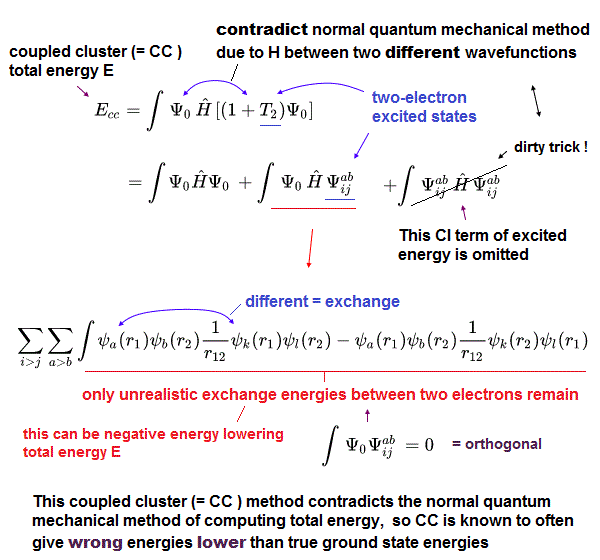
Instead of impractical time-consuming configuration interaction (= CI ), one of the impractical time-consuming approximations called coupled cluster (= CC or CCSD ) theory is often used.
The point is this coupled cluster (= CC ) theory contradicts the original Schrödinger equation, and can give wrong energies lower than the true ground-state lowest energies, which means CC is intrinsically a false theory (= CC is Not variational method, though the orthodox quantum variational method is also wrong ).
This-(6.30) says -- CC = Not variational
"The result is a set of equations which yield an energy that is not necessarily variational (i.e., may Not be above the true energy)" ← Coupled cluster (= CC ) is false.
This p.1-left-1st-paragraph says -- CC is wrong
"it is worth noting that
CCSD (= coupled-cluster with singles and doubles ) is Not variational, and it is also Not guaranteed
to give real-valued energies"
This p.16-top says -- CC wrong energy
"Consequently, the total CC energy is Not necessarily above the
exact ground-state total energy, just as in perturbation theory"
In CC, total energy (= E ) is given in a wrong way putting Hamiltonian energy equation (= H ) between two different wavefunctions like E = ∫Ψ0HΨab (← Not a normal ∫Ψ0HΨ0 or ∫ΨabHΨab, this p.18-(3.15), this p.27-2.29, p.29-2.40, this-6.28 ), which is the trick of CC lowering the total energy illegitimately faster than CI.
The (fake) total energy of the illegitimate CC can be lowered by the unphysically-negative exchange energy between two electrons of ground state and excited state (= ∫ψaψb1/r12 ψkψl, this p.8, this-p.7-lower, this-lower, this-(4) ) like CI.
↑ This means CC deliberately omits the annoying positive excited energy part to illegitimately lower the total energy than CI.
So CC is known to be able to get total energy lower than true ground state energy (= CC is Not variational ), which fact invalidates CC method.
There are many cases where coupled cluster (= CC, CCSD, CCSDT ) disagreed with experimental results.
This p.5-3rd-paragraph says -- CC is wrong
"The CCSD model systematically overestimates all core excitation energies".
This p.36-3rd-paragraph says -- CC fails
"CC fails for systems
with strong static correlation"
This-abstract-last says -- CC wrong energy
"CCSD(T) overestimates the formation energy"
This p.6-left-2nd-paragraph says -- CC wrong energy
"CCSDT we observe partially strong overestimation of the
total energy"
(Fig.5) MP2 is not variational, because it can give wrong energies lower than true atomic energy illegitimately.
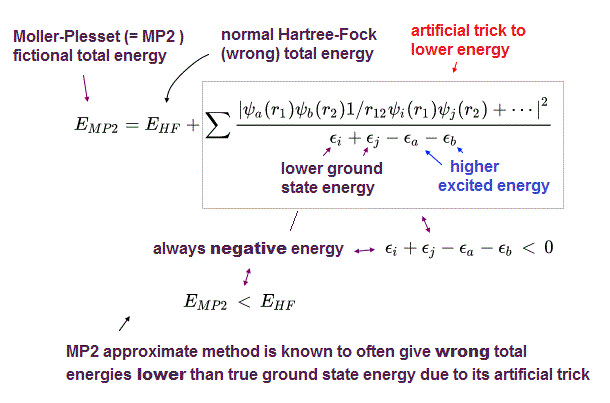
Instead of extremely-time-consuming configuration interaction (= CI ) and coupled cluster (= CC ), one of the illegitimate quantum mechanical approximations called Møller–Plesset perturbation theory (= MP) is often used to give the total energy lower than Hartree-Fock (= HF ) energy using deceitful tricks.
As shown in above figure, MP2 total energy (= EMP2 ) is always lower than Hartree-Fock energy (= EHF ) due to the artificially-introduced second term that is always negative.
The problem is this MP or MP2, which perturbation method (= PT ) is Not variational theory, which often gives wrong energies lower than true ground state energies (= overestimation of energies, this abstract-last, this p.7-2nd-paragraph ), which means Møller–Plesset perturbation theory is an unreal theory.
This p.12-last says -- MP2 wrong
"the MPn methodology is Not variational – it is possible that the
MP2 estimate for the correlation energy will be too large instead of too small."
This p.10-last says -- MP2 = Not variational
"MP theory uses a truncated Hamiltonian, and is therefore Not
variational. That means that one cannot use the calculated energy as a measure
of the quality of the calculation."
This or this-introduction-2nd-paragraph says -- MP2 fails
"Despite this popularity, the limitations of MP2-like methods are also well-known. In particular, they fail spectacularly for strongly correlated or metallic systems. Furthermore, a strong overestimation of dispersion interactions is observed for large polarizable systems"
This 1st-paragraph says -- MP2 wrong
"it suffers from the usual MP2 overestimation of dispersion."
This or this-p.2-2nd-last-paragraph says -- MP2 wrong
"Thus, the value of E(2), the first perturbation to the Hartree-Fock energy, will always be negative. Lowering the energy is what the exact correction should
do, although the Møller-Plesset perturbation theory correction is capable of overcorrecting it, since it is not variational"
↑ As shown here, Møller–Plesset perturbation theory (= MP2 ) is a defective illegitimate theory.
As a result, all the quantum mechanical methods are wrong, illegitimate and useless theories which must be replaced by the realistic simple useful atomic model.

Feel free to link to this site.