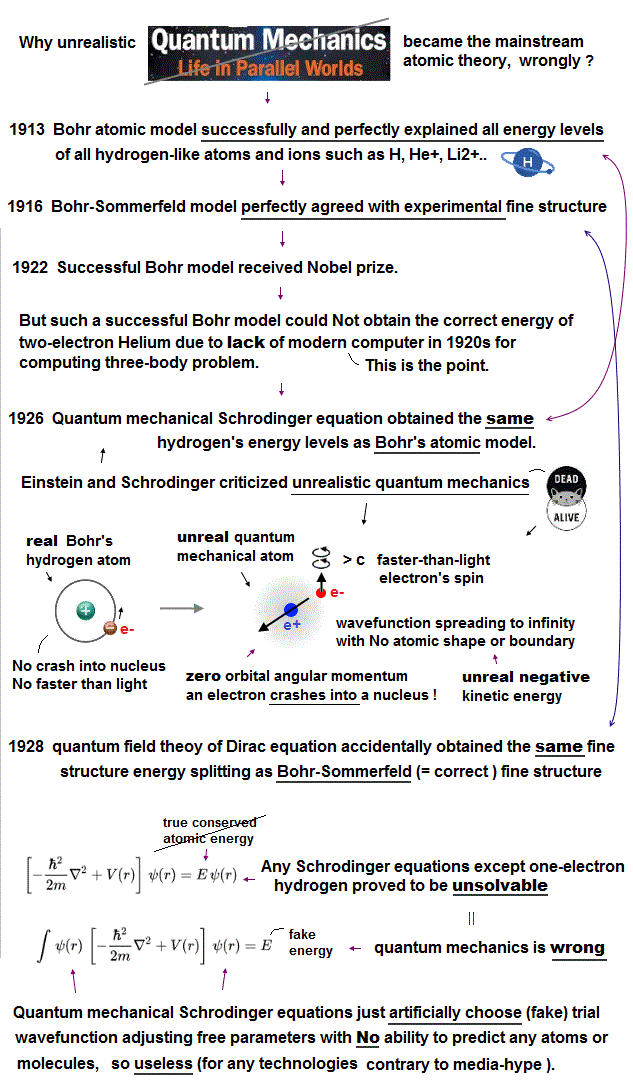
(Fig.1) Bohr's realistic atom perfectly agreed with experimental atomic energies and fine structure, but couldn't get correct helium atomic energy due to lack of modern computer in 1920s (= Not that Bohr's realistic model was wrong )

The current only mainstream atomic physics or quantum mechanics is extremely unrealistic based on fictional parallel universes, a dead-and-alive cat.. ← Why and where was the atomic physics forced to pick this wrong unphysical quantum mechanical theory ?
Even founders of quantum mechanics, Schrödinger and Einstein harshly criticized the quantum mechanics as "unrealistic".
Strangely, still Nobody understands such a crazy quantum mechanics even after its 100-year history, which facts strongly indicate there are obvious flaws in the present atomic theory or quantum mechanics, which must be replaced by more realistic atomic theory to advance science.
In 1910s, Bohr model successfully and perfectly explained all energy levels of all hydrogen-like atoms and ions such as H, He+, Li2+.. ( this p.3, this p.2 )
Contrary to the mainstream narratives, Bohr's realistic atom was stable, Not losing energy just by an electron orbiting around a nucleus, as shown in the fact that Bohr model was awarded Nobel prize as the legitimate mainstream atomic theory ( this last ).
The example of "accelerating charge radiating and losing energy by classical electromagnetism" uses a fictitious charged particle into which many smaller charges are packed against Coulomb repulsion, which does Not correspond to Bohr's realistic atom that uses a single unbreakable electron that does Not consist of smaller charges, and stable de Broglie wave.
Bohr's realistic atom used a real moving electron and the condition of the orbital length equal to an integral multiple of de Broglie wavelength (= avoiding destructive interference of electron's de Broglie wave ) to successfully obtain correct atomic energy levels, quantized angular momentum (= ℏ ), and experimentally-confirmed Bohr magneton (= magnetic field produced by Bohr's electron's orbital motion ), most of these Bohr atomic concepts are used also in quantum mechanics ( this p.15-(10-25), this p.3-left-middle, this p.11-last, this p.5-(c) ). = Bohr model is right.
This de Broglie wave interference of various particles was confirmed as real in many experiments such as Davisson-Germer.
Schrödinger equation = quantum mechanical only calculation tool accidentally obtained the same hydrogen's energy levels as the Bohr's model using the same principle of de Broglie wave theory ( this 2~3rd paragraphs ) after replacing Bohr's realistic electron's orbit with unphysical electron spin.
↑ Reason why unphysical quantum mechanical Schrödinger equations could give energies seemingly close to experimental values (= not in a legitimate way, though ) is that it uses the same de Broglie wave theory, Bohr magneton and radius as the successful Bohr's atomic model, Not because quantum mechanics itself was right.
Quantum mechanical atoms must always include unrealistic negative kinetic energy ( this p.8 ) and zero orbital angular momentum where an electron must always crash into a nucleus in its linear motion (= zero angular momentum ) which causes destructive interference of de Broglie wave, hence unstable ( this middle ). ← Quantum mechanical model has been false from the beginning.
Furthermore, in 1916, Bohr-Sommerfeld model perfectly and successfully explained the experimental fine structure energy splitting that was "accidentally" the same as the later quantum field theory or Dirac equation's hydrogen fine structure result. ← It means the quantum mechanics illegitimately and repeatedly copied the good points of the successful Bohr's atomic theory.
This p.14-4th and last paragraphs says -- Sommerfeld fine structure
"Sommerfeld’s fine-structure formula was spectroscopically confirmed for
H and for hydrogenic ions (He+,
Li2+, ...) to the highest precision—the greatest triumph
of the old quantum theory"
"Over a decade later, after the electron spin had
been discovered, relativistic quantum mechanics
(Dirac 1928) obtained the fine-structure formula (Gordon 1928) along a very
different route....
yields the same energies as Sommerfeld’s formula"
This-3rd-last-paragraph says, -- Dirac copied Sommerfeld
"When Dirac developed relativistic quantum mechanics, the relativistic Coulomb problem proved to be exactly solvable ...But the resulting formula for the energy levels was truly a surprise: The new answer was precisely the old Sommerfeld formula !"
Quantum mechanics proved to be wrong.
Quantum mechanical electron spin is unreal, Not a real spinning, because the tiny electron must be spinning much faster than light to get the observed magnetic moment.
Furthermore, electron spin (magnetic energy) cannot explain strong Pauli principle repulsion, ferromagnet, singlet-triplet energy splitting, and paradoxical spin-orbit interaction or Alkali anomalous Zeeman effect, intermolecular energy.
So quantum mechanics had to introduce unphysical exchange energy lacking exchange force, which needs unreal shapeless atomic model hampering today's technology.
But unfortunately, this Bohr's realistic model could not obtain the correct helium atomic energy, because there were No modern computers to compute the complicated helium's two electrons' motion that was an extremely difficult three-body problem, which was impossible to solve with the old pen and paper approach (= the simplest circular helium model disagreed with experiments due to its ignoring the destructive interference of two electrons' de Broglie waves ).
This is why physicists had to reluctantly accept unphysical quantum mechanics as the only mainstream theory. ← Quantum mechanics cannot give analytical solutions of three-body helium atoms, either ( this p.2-top ). But it could use (illegitimate) approximate method ( this p.2-7 ) choosing (fake) trial wavefunctions, which is the main culprit that prevents today's technological innovation.
Quantum mechanics can only show unrealistic explanation that tunnel may be caused by the physically-impossible negative kinetic energy ( this 1st-paragraph, this-intro-2nd-paragraph, this p.7-Fig.4 ) whose detailed mechanism is unknown and Not given by contradictory quantum mechanics ( this 2-3rd paragraphs ).
This electron's tunneling can be naturally explained by normal thermal fluctuation or real de Broglie wave's pressure (= de Broglie wave was proven to have enough power to push or affect an electron's motion as shown in in actually-verified interference experiments ) even without unrealistic quantum mechanical negative kinetic energy.
This unphysical quantum mechanical atomic wavefunction treated as vague electron's probability cloud has to always spread to infinity with no boundary, which means each single electron must always exist in all different places simultaneously like a dead-and-alive cat living in fictional parallel worlds, which is called superposition.
This is why even founders of quantum physics = Schrödinger and Einstein did Not accept such a ridiculous quantum mechanics as a real theory.
To explain two-slit interference experiment of a single electron, quantum mechanics needs unrealistic parallel worlds into which a single electron "splits" in order to pass multiple different slits simultaneously. ← ridiculous.
Einstein relativity rejecting the real medium is false.
↑ The unphysical static quantum mechanical probability wavefunctions must be replaced by real de Broglie wave moving with electrons in the real medium.
Quantum mechanics was not only unreal but also useless except for one-electron hydrogen that accidentally gives the same energy values as Bohr's atom, because all quantum mechanical Schrödinger equations for multi-electron atoms and molecules are unsolvable, unable to give right atomic energies.
Contrary to the media-hype, quantum mechanical Schrödinger equation for one-electron hydrogen molecule ion (= H2+ ) is also unsolvable ( this p.2 3rd-paragraph, this p.1-left-last-paragraph, p.2-right uses fictitious quasi-wavefunction, this p.11-5.conclusion ).
So physicists have to artificially pick up fake solutions called trial wavefunctions and basis sets out of infinite choices, and artificially manipulate many parameters, which quantum mechanical method cannot predict any atomic energies. ← Not science but just "art"
↑ There is No way of knowing what (fake) trial wavefunctions can give energies close to experimental values, until physicists waste much time to compare their calculation results with experimental energy values one by one ( this or this p.4 5th-paragraph, this 2nd-paragraph, this p.21-lower, this 3~5th paragraphs ).
So quantum mechanical calculation method is completely meaningless, it is far better to use the experimental energy values (= real atomic shapes ) from the beginning instead of wasting too much time in quantum mechanical meaninglessly-inefficient calculation of unsolvable Schrödinger equations that were unable to predict any physical values.
We proved that quantum mechanical Schrödinger equations for multi-electron atoms can never find true solutions like unsolvable H2+ molecular ion (= true atomic wavefunction must always conserve the constant total energy in any electrons' positions, which is proved to be impossible ), hence quantum mechanics is officially proven false.
Unlike the quantum mechanical wrong helium atomic model that cannot conserve total energy, the realistic Bohr's helium model, which gives perfectly correct helium's experimental energies, can precisely conserve total energy in any electrons' positions.
And this realistic Bohr's helium model whose Pauli principle is based on real de Broglie wave's destructive interference can use practical atomic model with actual shape that can advance the current stalled nano-technology.
Quantum mechanical unreal exchange energy prevents each atoms from having shapes, which hampers today's nano-technology
(Fig.2) Bohr's model's exact atomic energies and magnetic moment (= Bohr magneton )
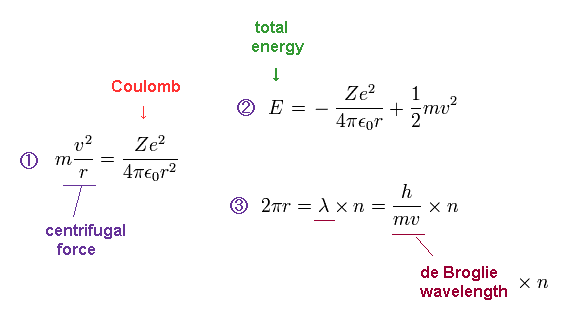
Bohr's realistic atomic model perfectly agreed with experimental energies of all the hydrogen-like atoms and ions (= H, He+, Li+.. ) by assuming an orbital length (= 2πr r is orbital radius ) equals an integer (= n ) times de Broglie wavelength (= λ = h/mv h is Planck constant, m is electron's mass, v is electron's velocity ).
Contrary to many wrong explanations, Bohr's atomic model was stable and consistent with experimental results (and results of Schrödinger equation's hydrogen atom, this p.9, this p.10-4.5.4 ), which is why Bohr model could obtain Nobel prize.
In Bohr model, the centrifugal force (= of electron's orbit of an integer times de Broglie wavelength λ ) equal to Coulomb electric force from Ze+ nucleus gives the total energy E which is the sum of Coulomb potential energy and kinetic energy ( this p.3 ). ← A hydrogen ground-state energy E = -13.6 eV ( Z = 1, n = 1 ).
de Broglie wavelength was proven to be real in many experiments such as Davisson-Germer experiments.
Bohr model could naturally obtain the experimental hydrogen magnetic moment called Bohr magneton (= μB ) that accidentally agreed with the unrealistic electron spin, and Bohr radius.

Feel free to link to this site.