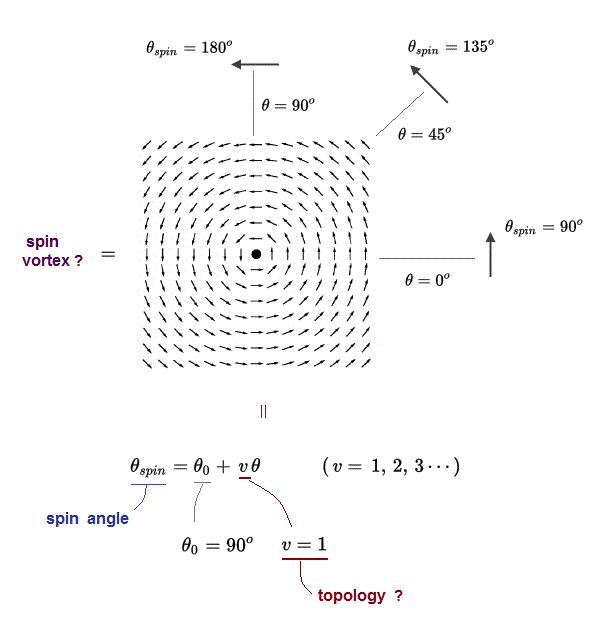
(Fig.1) Electron spin is unreal, needing faster-than-light spinning, and unable to explain Pauli principle, ferromagnet. ↓

Quntum mechanical Schrödinger equations are unsolvable (= having No analytical solution ) except for one-electron hydrogen atom ( this p.21-upper ) whose energy agreed with Bohr's (realistic) atomic model.
The problem is this quantum mechanical hydrogen atom always includes unrealistic s orbital with zero orbital angular momentum (= ground state ) that doesn't produce the observed magnetic moment.
So quantum mechanics had to introduce the (fictional) electron spin model (= unreal, this or this ) to explain the observed magnetic moment of hydrogen atom, which is (accidentally) equal to Bohr magneton (= μB ) of the realistic electron's orbit ( this p.2, this 3rd-paragraph, this p.3 ).
But the tiny electron (= whose maximum size can be estimated by Coulomb scattering between particles ) must be spinning much faster than light to generate this observed magnetic moment (= Bohr magneton μB ) or spin angular momentum 1/2ℏ ( this 3rd-paragraph ).
So electron spin is unreal, an electron is Not actually spinning (= which is the current official conclusion ), contrary to the media's misleading electron spinning picture.
This 3rd-paragraph says
"physicists soon determined that an electron would have to be spinning much faster than the speed of light to produce the effects they were seeing" ← Electron spinning is unreal.
And this electron spin's magnetic ( spin-spin dipole-dipole ) energy (= only 10-4 or 0.0001 eV, this p.10-left-2nd-paragraph, this p.17 ) is too weak, unable to explain strong Pauli principle repulsive energy (= more than 10eV is needed to exclude the 3rd electron from inner occupied n=1 orbital to the outer n = 2 orbital in lithium ), ferromagnetism (= ~ 1eV ), the singlet-triplet states' energy difference (= ~ 3eV, this p.8(or p.5)-upper, this p.7 ).
This p.13-lower says "Indeed, the Pauli equation does Not provide any insight into the origin or characteristics of spin: Pauli's theory does Not explain the origin of the spin, nor does it give any reason for its magnitude." ← Pauli principle is completely irrelevant to the (unphysical) spin with No proof ( this p.60-3, this p.1-right-1st-paragraph ).
The unphysical electron spin also cannot explain alkali anomalous Zeeman effect based on paradoxical spin-orbit interaction (= fine structure energy splitting can be perfectly explained by the realistic Bohr-Sommerfeld model without spin or spin-orbit interaction, this-lower, this p.14 )
(Fig.2) Pauli principle by unphysical antisymmetric wavefunction causes unreal shapeless (= borderless ) atoms hampering technology. ↓
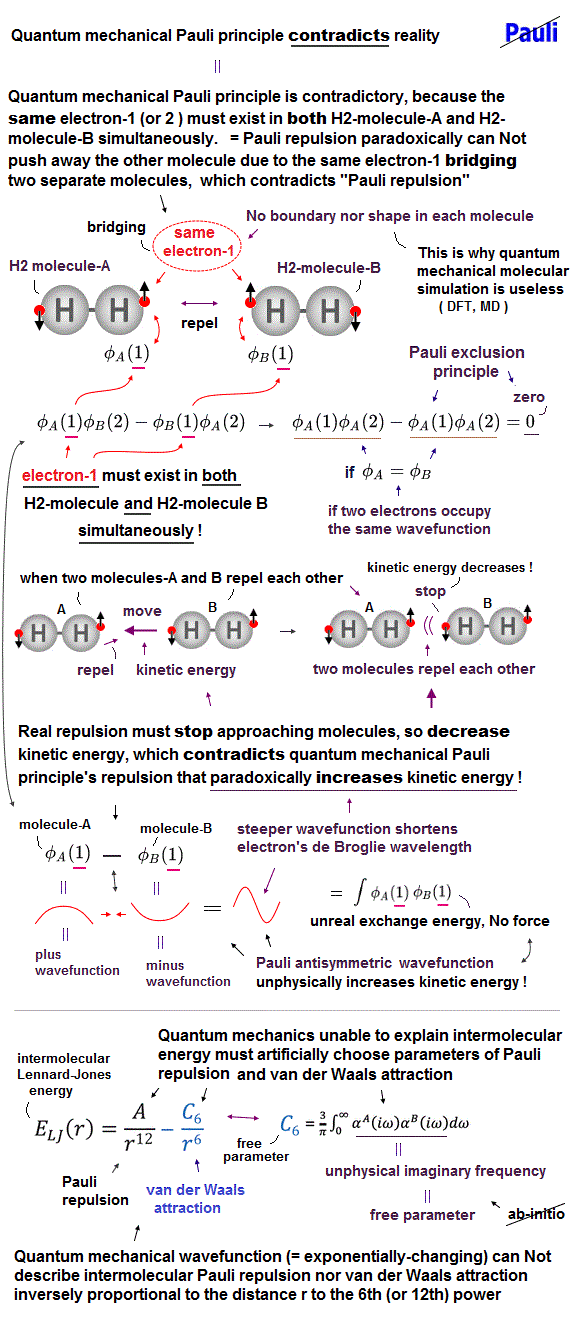
The original (unphysical) electron's spin failed to explain the strong Pauli exclusion principle or repulsive energy.
So physicists had to create an artificial ad-hoc concept called exchange energy to explain Pauli principle, ferromagnet and singlet-triplet energy splitting ( this p.6, this p.7-8, this p.7 ), all of which are actually caused by real forces such as Coulomb electric interaction, Not by the unrealistic electron spin.
This ad-hoc exchange energy is unrealistic, lacking real exchange force ( this p.9-upper, this p.5-1st-paragraph, this-middle-exchange energy ), contradicting the real energy that should be equal to force × distance.
Pauli principle repulsion based on such an unrealistic exchange energy is considered to be Not a real force, which contradicts the fact that Pauli repulsion can be felt as real repulsive contact (or normal ) force, when we touch some objects.
↑ Because the unphysical quantum mechanical Pauli repulsion is caused by (pseudo-)kinetic energy change instead of real force potential energy change.
According to such a paradoxical quantum mechanics, neither this strange exchange energy nor Pauli repulsion has real physical meaning ( this p.11, this p.8, this p.3 ).
This p.6 says "The Pauli Exclusion Principle is one of the basic principles of modern physics... it is still debated today because an intuitive, elementary explanation is still missing"
All quantum mechanical Schrödinger equations for multi-electron atoms and molecules are unsolvable, useless, wrong.
Physicists have to artificially choose fake trial wavefunctions or basis sets which must take the form of (unphysical) antisymmetric wavefunction (= or Slater determinant, this p.9-12 ) for expressing (unphysical) Pauli exclusion principle ( this p.3-9.6, this p.2-1st-paragraph ).
Exchanging any two electrons (= two electrons' labels ) is required to flip the sign of this unphysical antisymmetric wavefunctions.
So if the antisymmetric wavefunction includes the same two orbitals, the whole antisymmetric wavefunction becomes zero, which mathematical abstract unphysical relation is supposed to mean (unreal quantum mechanical) Pauli exclusion principle.
The problem is that in this unphysical antisymmetric wavefunction, every electron must always unrealistically exist in all different orbitals or different atoms simultaneously due to exchanging all electrons among all different atomic orbitals ( this p.11, this p.15-upper, this p.3-last ).
This p.3-2nd-paragraph says
"The terms 'first electron' and 'second electron' have no meaning as
the electrons are indistinguishable and it is never possible to put a label (= atomic orbitals ) on one specific electron"
↑ It means a single electron always spreading over all different atomic orbitals forbids each atom from having clear boundary or shape.
This is why quantum mechanical atoms are unrealistically shapeless, and density functional theory (= DFT ) approximation treating the whole material as one pseudo-electron model is the current mainstream quantum mechanical method.
This unphysical quantum mechanical shapeless atomic model based on paradoxical Pauli exchange antisymmetric wavefunction is the main culprit of hampering scientific development through impractical DFT and unrealistically-time-consuming molecular dynamical (= MD ) simulation.
Furthermore, this Pauli antisymmetric wavefunction is unable to correctly describe more than two electrons mixing singlet and triplet states, so quantum mechanical Pauli principle proved to be false.
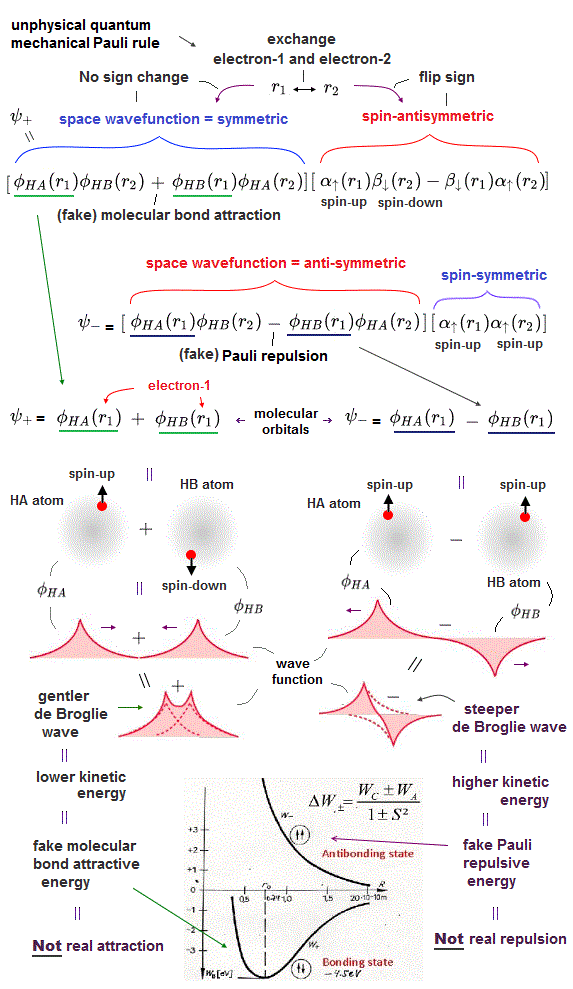
(Fig.3) gentler (or steeper anti-)symmetric wavefunction decreases (or increases ) electron's kinetic energy by longer (or shorter ) de Broglie wavelength, which is the origin of the unphysical quantum mechanical Pauli principle.
This unphysical exchange energy is necessary to explain the unphysical quantum mechanical molecular bonds (= symmetric, singlet ) and Pauli repulsion (= antibond, antisymmetric, triplet ), because quantum wavefunction's Coulomb electric energy alone is too weak to form molecular bonds ( this p.3-4, this p.3 ).
In the molecular attractive bond, this unphysical exchange energy can artificially lower electrons' (pseudo-)kinetic energy (= which lowers total energy ) to cause the (fake) molecular bond ( exchange ) energy.
This p.6-1st-paragraph says
"clear indication that bonding takes place because of the decrease in the kinetic energy
This is the conclusion that was reached by
Hellmann in 1933 and also by Feynman"
Some textbooks often falsely say the molecular attractive bond energy may be caused by higher electron's probability density between two atoms due to symmetric wavefunction.
↑ Whether the electron's probability density becomes higher between two atoms or nor is irrelevant to molecular bond energy, because if the total energy is conserved, electrons in any places (= whether electrons are between two atoms or not ) must have the same total energy by adjusting kinetic energy to Coulomb potential energy.
So the quantum mechanical attractive molecular bond energy is caused by the decrease in electron's kinetic energy against the Coulomb potential energy by violating total energy conservation law (= quantum mechanical fake molecular bond is due to decreasing kinetic energy of electrons even in lower Coulomb potential energy region between two atoms violating total energy conservation ).
This quantum mechanical molecular bond based on the decreased kinetic energy is unreal, wrong, contradicting the fact that the real attractive force should increase the electron's kinetic energy (= due to real attraction pulling and accelerating electrons increasing kinetic energy ) instead of decreasing.
In the same way, quantum mechanics says Pauli repulsive energy (= contact force ) is caused by increased kinetic energy of electrons ( this p.27-1st~2nd-paragraph, this introduction-3rd-paragraph ) expressed as unphysical exchange energy in antibond or antisymmetric wavefunctions.
↑ The curvature of the antibond or antisymmetric wavefunction increases, which shortens the electron's de Broglie wavelength and increases the kinetic energy (= which increases total energy ), which is the origin of the unphysical quantum mechanical Pauli repulsion ( this p.13(or p.12)-2nd-paragraph ) lacking real force ( this p.9-10 ).
This p.4(or p.3)-2nd-paragraph says
"The increase in
slope causes an increase in kinetic energy, which reflects the Pauli repulsion"
This quantum mechanical Pauli repulsion based on the increased kinetic energy is also unreal and wrong, because the real repulsion should repel or decrease (= decelerate ) the electrons' kinetic energy.
As a result, the quantum mechanical molecular bonds and Pauli repulsion based on the paradoxical exchange energy lacking real force disproves the quantum mechanics.
(Fig.4) Intermolecular (= Lennard-Jones potential ) energy parameters must be empirically adjusted instead of (useless) quantum mechanical prediction.
In fact, this quantum mechanical Pauli repulsive exchange energy can Not explain the actually-observed Pauli repulsive energy.
Pauli repulsion is known to contribute to the repulsive energy in the intermolecular (= dispersion ) energy called Lennard-Jones potential ( which represents Pauli repulsive contact force when you touch hard objects without causing covalent bonds between your finger and objects ).
This intermolecular Lennard-Jones potential consists of weak van der Waals attraction and strong Pauli repulsion.
Quantum mechanical exchange energy (= Pauli repulsive energy and molecular bond energy ) is roughly proportional to exponential function divided by the intermolecular distance (= r ) like e-α r /r ( this p.2-right-A. ), which can Not explain the actual van der Waals attraction proportional to 1/r6 nor Pauli repulsion proportional to 1/r12 ( this p.4-2nd-paragraph, this p.2-left-1st-paragraph ).
↑ In the intermolecular potential, van der Waals attraction is proportional to -1/r6 (= r is distance between two molecules ), so if Pauli repulsive potential is proportional to e-αr/r as quantum mechanical wavefunction demands, the total intermolecular potential unrealistically diverges to negative infinity in r → 0 (= quantum mechanical unreal Pauli repulsive potential of e-αr/r can Not cancel the actual van der Waals potential of -1/r6 in r → 0 ), which means the distance between two molecules must be unrealistically zero (= negatively infinite potential energy is the most stable ) against the actual finite intermolecualr distance (= r = finite ), which means quantum mechanics can Not describe actual Pauli principle repulsion.
So physicists have to artificially and empirically adjust intermolecular energy parameters (= for Pauli repulsion and van der Waals attraction ), which is Not quantum mechanical prediction ( this p.3, this p.2-right-last, this p.45-2.7.2, this p.11-(32)(33) ).
This 5th-paragraph says
"The form of the repulsion term has No theoretical justification; the repulsion force should depend exponentially on the distance, but the repulsion term of the L-J (= Lennard-Jones ) formula is more convenient due to the ease and efficiency of computing r12"
As a result, the quantum mechanical Pauli principle exchange energy is not only unphysical but also inconsistent with the quantum mechanical prediction itself ( this p.11, this p.19-1st-paragraph ), hence wrong.
The present mainstream quantum mechanical DFT approximation also just chooses artificial intermolecular (= dispersion ) pseudo-potential parameters whose parameters can be adjusted freely (= empirically ), which can Not predict any intermolecular Pauli repulsion nor van der Waals attraction.
Quantum mechanics and (paradoxical) Einstein relativity prohibit the existence of any real substance in space ( except for the charged particles such as electrons and nuclei ).
↑ Quantum mechanics refuses to admit that experimentally-observed electron's de Broglie wave or wavefunction causing interference is real wave or substance, which unreasonable quantum mechanical interpretation (= No real substance for expressing Pauli principle repulsion ) is the root cause of its unphysical Pauli principle and the current stalled science, which must be replaced by real atomic model based on real substance generating real Pauli repulsion (= caused by de Broglie wave destructive interference ).

Feel free to link to this site.