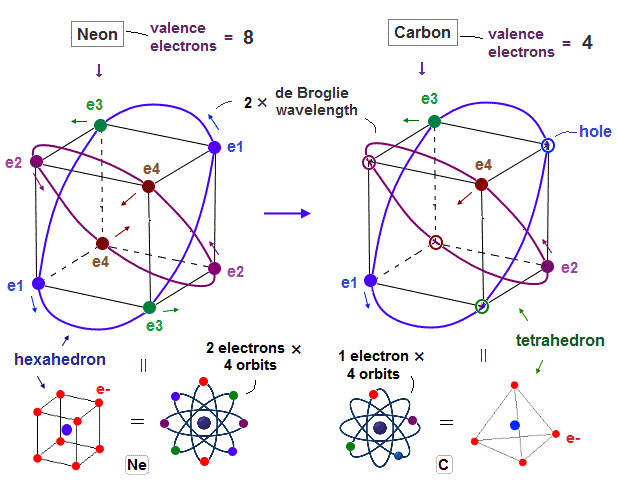
(Fig.1) Carbon with 4 valence electrons (= 2 electrons in 2s, 1+1 in 2p ? )

Carbon (= C ) has 4 valence electrons. According to quantum mechanics, Carbon's 2 electrons in 2s orbital and 1 electron in each 2p orbital.
The point is these quantum orbitals cannot be seen, so they are just "imaginary" things. Electron spin is faster-than-light, lacking reality.
They claim each orbital can have up to two electrons of spin up and down. But too weak spin disagrees with Pauli exclusion principle.
So this Cabon model by quantum mechanics is unreal and inconsistent. We need realistic and consistent atomic model !

2016/10/14 updated. Feel free to link to this site.