





 |
|



|
Molecular Association and Thermoreversible Gelation
|
|
 Phase Formation of Water-Soluble Associating Polymers Phase Formation of Water-Soluble Associating Polymers
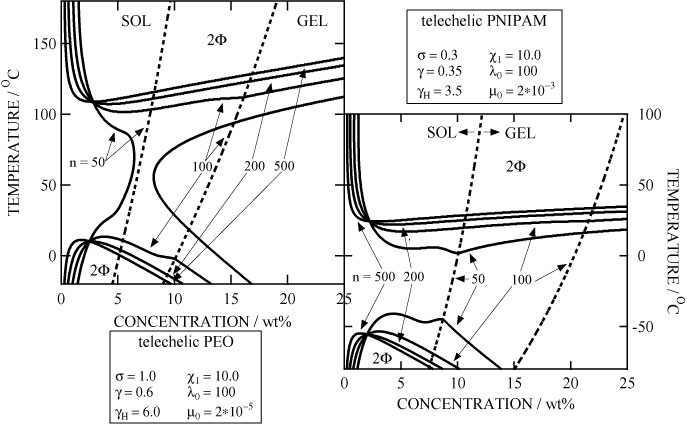 We theoretically study interference between end-chain association and main-chain hydration in solutions of hydrophobically modified telechelic water-soluble polymers such as Hydrophobic Ethoxylate Urethane (HEUR), Hydrophobic Poly(N-isopropyl acrylamide) (HM-PNIPAM) on the basis of "Theory of Associating Polymer Solutions", and derive the phase diagrams showing thermoreversible gelation and LCST phase separation. We also study cooperative hydration (hydrogen-bonded water molecules with positive correlation) of temperature-sensitive water-soluble polymers by incorporating the nearest neighbor interaction among the bound water molecules. Statistical-mechanical study of the thermoreversible sol/gel transitions with multiple junctions in polymer solutions, polymer-surfactant interactions as seen in aqueous solutions of associating polymers are also studied. We theoretically study interference between end-chain association and main-chain hydration in solutions of hydrophobically modified telechelic water-soluble polymers such as Hydrophobic Ethoxylate Urethane (HEUR), Hydrophobic Poly(N-isopropyl acrylamide) (HM-PNIPAM) on the basis of "Theory of Associating Polymer Solutions", and derive the phase diagrams showing thermoreversible gelation and LCST phase separation. We also study cooperative hydration (hydrogen-bonded water molecules with positive correlation) of temperature-sensitive water-soluble polymers by incorporating the nearest neighbor interaction among the bound water molecules. Statistical-mechanical study of the thermoreversible sol/gel transitions with multiple junctions in polymer solutions, polymer-surfactant interactions as seen in aqueous solutions of associating polymers are also studied.

|
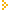 Rheology of Transient Networks Rheology of Transient Networks
 Dynamics of association and dissociation processes in polymer networks with micellar junctions in solutions of telechelic associating polymers are theoretically studied on the basis of "Nonaffine Transient Network Theory" . Fluctuations of the junctions, their diffusive motion, nonlinear viscosity (shear thickening), dynamic-mechanical moduli, stress relaxation, flow hardening, stress overshoot are studied. The results are compared with experiments on HEURs and telechelic PNIPAMs. Dynamics of association and dissociation processes in polymer networks with micellar junctions in solutions of telechelic associating polymers are theoretically studied on the basis of "Nonaffine Transient Network Theory" . Fluctuations of the junctions, their diffusive motion, nonlinear viscosity (shear thickening), dynamic-mechanical moduli, stress relaxation, flow hardening, stress overshoot are studied. The results are compared with experiments on HEURs and telechelic PNIPAMs.

|
 Theoretical Study of Thermoreversible Gelation Theoretical Study of Thermoreversible Gelation
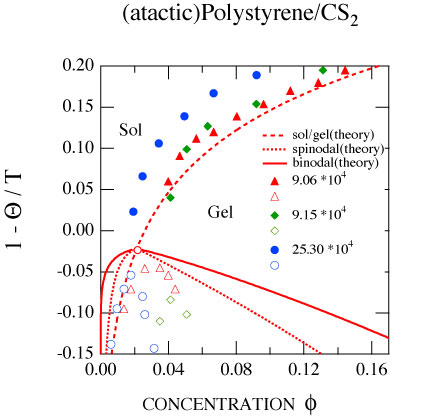 Sol-gel transition with multiple junctions, gelation of helix-forming biopolymers, of multicomponent mixed networks, high-temperature gelation with polymer conformation changes are studied from a unified model of thermoreversible gelation. It is shown that thermoreversible gelation is a phase transition similar to the Bose-Einstein condensation: condensation of polymers in momentum space (not in the position space) into a network with no center-of-mass translational degrees of freedom. Sol-gel transition with multiple junctions, gelation of helix-forming biopolymers, of multicomponent mixed networks, high-temperature gelation with polymer conformation changes are studied from a unified model of thermoreversible gelation. It is shown that thermoreversible gelation is a phase transition similar to the Bose-Einstein condensation: condensation of polymers in momentum space (not in the position space) into a network with no center-of-mass translational degrees of freedom.
|
|
Hydrogen-Bonded Supramolecules
|
 Thermoreversible Gels with Hydrogen-Bonded Zipper-like Cross-links Thermoreversible Gels with Hydrogen-Bonded Zipper-like Cross-links
 Structure and rheology of networks cross-linked by zipper-like sequential hydrogen bonds, such as seen in helical junctions in biopolymer gels and polymer complexes, are studied by statistical-mechanical theory and molecular simulation. Chiral order induced by hydrogen bonds, helical polymers formed by hydrogen-bonding chiral molecules are also studied. The figures show snapshots of networks at 3 different temperatures, whose junctions consist of hydrogen-bonded ladder-like pairs of chain segments. Structure and rheology of networks cross-linked by zipper-like sequential hydrogen bonds, such as seen in helical junctions in biopolymer gels and polymer complexes, are studied by statistical-mechanical theory and molecular simulation. Chiral order induced by hydrogen bonds, helical polymers formed by hydrogen-bonding chiral molecules are also studied. The figures show snapshots of networks at 3 different temperatures, whose junctions consist of hydrogen-bonded ladder-like pairs of chain segments.

|
 Hydrogen-Bonded Liquid Crystals Hydrogen-Bonded Liquid Crystals
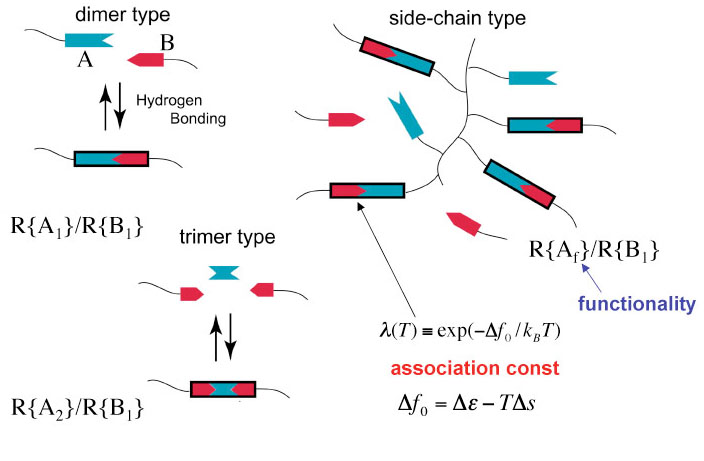 Some rigid molecules are known to undergo liquid crystallization when hydrogen-bonded to each other. For a binary mixture of low-mass molecules, as well as polymers, R{A} and R{B}, each carrying at least one rigid part A and B that form mesogenic core when associated. Dimer type, trimer type, main-chain type, side-chain type, combined type, and network type are known. These are called hydrogen-bonded supramolecular liquid crystals. For example, aromatic acid derivatives with alkoxy or alkyl terminal groups form dimers due to hydrogen bond between their carboxylic acid groups, and show mesomorphism. Association between different species of molecules also induces the isotropic/anisotropic phase transition. The most remarkable case is that the non-mesogenic molecules form compounds with mesogenic cores when hydrogen-bonded. In such combinations of molecules, isotropic materials undergo liquid crystallization by simple mixing. Some rigid molecules are known to undergo liquid crystallization when hydrogen-bonded to each other. For a binary mixture of low-mass molecules, as well as polymers, R{A} and R{B}, each carrying at least one rigid part A and B that form mesogenic core when associated. Dimer type, trimer type, main-chain type, side-chain type, combined type, and network type are known. These are called hydrogen-bonded supramolecular liquid crystals. For example, aromatic acid derivatives with alkoxy or alkyl terminal groups form dimers due to hydrogen bond between their carboxylic acid groups, and show mesomorphism. Association between different species of molecules also induces the isotropic/anisotropic phase transition. The most remarkable case is that the non-mesogenic molecules form compounds with mesogenic cores when hydrogen-bonded. In such combinations of molecules, isotropic materials undergo liquid crystallization by simple mixing.

|
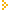 Helical Polymers induced by Hydrogen Bonding of Chiral Molecules Helical Polymers induced by Hydrogen Bonding of Chiral Molecules
 The effect of stretching polymer chains bearing helical segments induced by hydrogen-bonded chiral molecules in the mixtures of chiral molecules is theoretically studied. The end-to-end distance R of a polymer with induced helical sequences and the chiral order parameter \psi (difference in the content of left- and right-handed helices on a chain) are calculated as functions of the external tension given at the chain ends. The chiral order parameter is shown to be sensitive to chain extension and drastically enhanced by stretching. With a slightest enantiomer excess, therefore, a mixture of chiral molecules in a solution can be separated into assemblies of pure chirality by repeating the operation of stretching polymers and washing the adsorbed chiral molecules. Possibility of optical resolution of racemic solutions by stretching polymeric gels consisting of such hydrogen-bonded helical subchains is discussed. The effect of stretching polymer chains bearing helical segments induced by hydrogen-bonded chiral molecules in the mixtures of chiral molecules is theoretically studied. The end-to-end distance R of a polymer with induced helical sequences and the chiral order parameter \psi (difference in the content of left- and right-handed helices on a chain) are calculated as functions of the external tension given at the chain ends. The chiral order parameter is shown to be sensitive to chain extension and drastically enhanced by stretching. With a slightest enantiomer excess, therefore, a mixture of chiral molecules in a solution can be separated into assemblies of pure chirality by repeating the operation of stretching polymers and washing the adsorbed chiral molecules. Possibility of optical resolution of racemic solutions by stretching polymeric gels consisting of such hydrogen-bonded helical subchains is discussed.
|
|