
教材出所:

1.Smokers' rights: for or against?
TV anchor:
One local government is planning to ban smoking in public places
such as hotels, restaurants and the like. Owners of restaurants
and hotels fiercely object to the plan. As a compromise, they may
be allowed to have enclosed smoking sections.
John:
What do you think of this issue?
Diana:
Well, separating smoking and nonsmoking sections seems to be
a good idea. But, even so, some people will still have to work
in an unhealthy environment. For example, workers in
restaurants have to serve customers in smoking sections.
John:
That's a bit extreme, isn't it? If you insist on such a point,
no compromise can be made. If we admit what you say is a valid
argument, we will have to ban smoking in public places
completely. I can't support that.
Diana:
I don't think you get my point. Nobody has the right to endanger
nonsmokers' health.
John:
I disagree. Smokers' rights should be protected, too. I mean
both smokers' rights and nonsmokers' rights should be
protected equally, at least in public places.
Diana:
Listen, we used to accept smokers' rights, but their manners
are terrible.
For instance, I may be exaggerating, but smoking cigarettes
while walking results in the burning of clothes and children's
faces, and many smokers throw cigarette butts in the road.
Besides, the majority of smokers don't even care about
the hazards of second hand smoke to nonsmokers.
John:
I see what you mean. In a sense, smokers forfeit their own rights
by violating the rights of nonsmokers. To put it differently, you
believe nonsmokers' rights are more important, right?
Diana:
Absolutely right.
2.Legalizing casinos : for or against?
TV anchor:
This year, a blitz of new Las Vegas-style casinos made Macao
the world's top gambling hub in revenue.
In Macao, there are so many rich people that money
no longer seems like money there.
Diana:
Did you know the revenue of casinos in Macao exceeded that
of Las Vegas this year?
John:
No. I was unaware of that. Businesses inLas Vegas seem to be
much bigger than those in Macao. Besides, Las Vegas not only
has casinos, but also other forms of entertainment such as
exhibitions, shopping malls, hotels and big-name shows.
I'm surprised to find out that Macao takes in more money.
Diana:
Me, too. Speaking of casinos, are you for legalizing casinos
in Japan?
John:
Yes, I am, as a matter of fact. I think people nowadays get
stressed out from work. Gambling can be a good way to relax.
Once refreshed, they will be more motivated to work.
In this respect, I agree that casinos should be legalized.
Diana:
Actually, I don't agree with you. Japan already has legalized
gambling in horse and bicycle racing. Why-do we have to have
more gambling? A lot of people, especially the poor, tend to
gamble more money than they earn. They get addicted to
gambling and stop working. Most of them have families,and
sooner or later, they may suffer from a family breakdown or
become social dropouts.
John:
Don't only look at the bad sides of gambling! Casinos create new
businesses and more jobs. Even if people lose some money,
they have other chances to earn more money.
Diana:
You're missing the big picture. I have seen casinos cause some
people to gamble away not only their money but also their lives.
3.Cosmetic surgery : for or against?
TV anchor:
South Korea's reputation regarding cosmetic surgery
is well known. According to a survey, 60 percent of
respondents aged 18 to 24 have had cosmetic surgery.
In fact, it's very common for graduating students to have
surgery before job hunting.
John:
Do you agree with the practice of cosmetic surgery?
Diana:
Don't ask me such a question! I'm satisfied
with the way I look. This is me. I am
comfortable with who I am. What about you?
John:
Me? I don't need a perfect face. I am, however,
not 100% satisfied with the way I look.
Diana:
Do you want to have some changes made to
your face?
John:
To some extent, yes. For instance, I want to remove
my crow's feet, and I want to enlarge the size of my eyes.
Then I don't think I would be so concerned about my
appearance. Such surgery not only makes one look better,
but also boosts one's confidence. I think making small
changes is acceptable, like enlarging the size of one's eyes.
Diana:
I don't agree with you.inherited from our ancestors.
Our bodies are part of our identity. We should love
the way we are.
John:
Don't you understand why students in Korea decide
to have cosmetic surgery before job hunting?
Companies tend to hire the better-looking applicant
if two applicants have nearly identical abilities.
They don't want to lose opportunities due to their
unattractive traits.
Diana:
Just remember, after such surgery, your face
is changed forever.
4.A Compulsory voting system
TV anchor:
The Japanese Diet election took place last Sunday.
According to the election board, the final voting rate
was about 53%.
It was one of the lowest voter turnouts of the past two
decades. The election board is discussing what Japan
should do to raise voters' interest in elections.
Diana:
Why don't people vote in elections?
John:
Many of them are not very interested in politics, I suppose.
To make matters worse, they think politics is none of
their business.
Diana:
Really? I'm surprised. The right to vote is given to anyone
20 years old and over, isn't it?
It's strange for them to abstain from this right.
John:
People have the right to vote, but it's not compulsory in Japan.
Diana:
In order to raise voting rates, isn't it a good idea to fine people
who don't vote in elections?
John:
I don't think so. To reiterate, it's not compulsory.
Moreover, voting rates are not always this low.
Raising voting rates is not necessarily directly related to
raising voters' interest in politics.
Diana:
I think you are wrong on that point. Even if people are
being made to vote in elections, they might become more
interested. Besides, voting in elections doesn't only mean
choosing members of the Diet, but also means sharing
their opinions with others.
John:
I understand your point. However, I still feel uncomfortable
fining people who don't vote.
CurrentTopics & スタ-リンのソ連軍






ロシアで“感染”再拡大…
連日2万人超える新規感染者
2021年6月30日放送「news every」
ロシアで新型コロナウイルスの新規感染者数が再び急増している。
感染力が強いとされるインド型の変異ウイルスが猛威をふるって
いるとみられ、24日には5カ月ぶりに2万人を突破した。
世界で初めて新型コロナのワクチンを承認したロシアだが、
急速な感染拡大で経済や政治への影響も懸念される。
政府当局によると、24日の新規感染者数は2万182人となった。
2万人台となったのは1月24日以来。
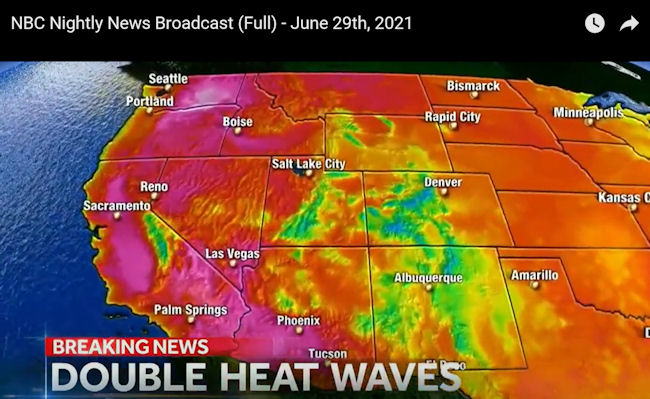
US and Canada heatwave
July 1 2021,Thursday
Pacific Northwest sees record temperatures.
The US National Weather Service has issued heat warnings
for much of Washington and Oregon states. Parts of
California and Idaho are also affected.
The temperatures in Seattle, Washington State, reached
101F (38C), a record in the city for June.
Some cities have opened cooling centres, where residents
can escape the heat in air-conditioned buildings.
The soaring temperatures are due to a dome of high
pressure hovering over north-western United States and
Canada.
Experts say climate change is expected to increase
the frequency of extreme weather events, such as
heatwaves, however linking any single event to global
warming is complicated.
As the climate changes, there could be an increase
in the number of deaths from floods, storms and
heatwaves, experts say.
The National Weather Service (NWS) said that even
hotter temperatures were forecast for the coming days
throughout the Pacific Northwest as well as parts of
western Nevada and California.
Despite the warnings many people have been enjoying
the sunshine, with lakes busy and pools running at full capacity.
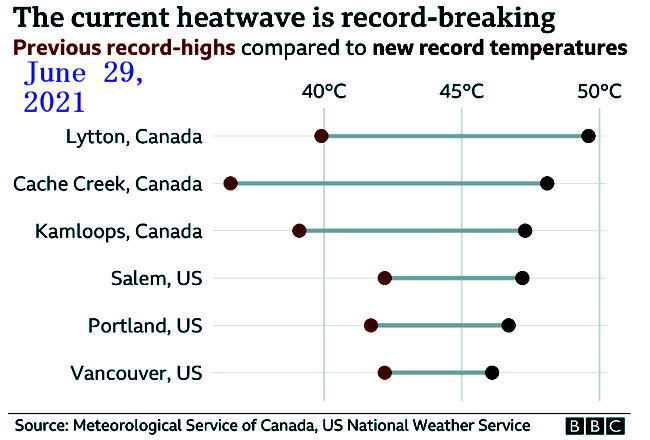
Historic Northwest heat wave
may have killed hundreds
July 2, 2021, Friday
Hundreds of deaths in Canada, Oregon and Washington may have been
caused by the historic heat wave that baked the Pacific Northwest and
shattered all-time temperature records in usually temperate cities.
Oregon health officials said late Wednesday more than 60 deaths have been
tied to the heat, with the state's largest county, Multnomah, blaming
the weather for 45 deaths since the heat wave began Friday.
British Columbia's chief coroner, Lisa Lapointe, said her office received
reports of at least 486 "sudden and unexpected deaths" between
Friday
and Wednesday.
Normally, she said about 165 people would die in the province
over a five-day period.
"While it is too early to say with certainty how many of these deaths
are
heat related, it is believed likely that the significant increase in deaths
reported
is attributable to the extreme weather,"
Lisa LaPointe said in a statement. Like Seattle, many homes
in Vancouver, British Columbia, don't have air conditioning.
"Vancouver has never experienced heat like this, and sadly dozens
of
people are dying because of it," Vancouver police Sgt. Steve Addison
said.
Washington state authorities had linked more than 20 deaths to the heat,
but that number was likely to rise.
The heat wave was caused by what meteorologists described as a dome of
high pressure over the Northwest and worsened by human-caused climate
change, which is making such extreme weather events more likely and more
intense.
Seattle, Portland and many other cities broke all-time heat records,
with temperatures in some places reaching above 46 C.

Japan's total population
June 25, 2021
The latest census was conducted in October 2020,
and the preliminary results were released Friday.
The Ministry of Internal Affairs and Communications said
Japan's total population in 2020 was 126,227,000,
a decrease of 868,000, or 0.7 percent, over the 2015 census.
Tokyo's population was 14,065,000, the first time the figure
in the capital topped 14 million and representing a surge of
549,000 residents over the 2015 census, the largest increase
for any prefecture.
The other eight prefectures that recorded population gains
were Kanagawa, Saitama and Chiba, which all neighbor Tokyo,
as well as Aichi, Fukuoka, Okinawa, Osaka and Shiga.
However, the increase in Shiga Prefecture was only 1,000,
while Osaka Prefecture's population rose by just 3,000.
The 38 prefectures that lost population were mainly rural areas.
Hokkaido had the largest decrease at 153,000.
Population declines were also recorded in 1,416, or 82.4 percent,
of Japan's 1,719 municipalities. More than half of
the municipalities saw their populations shrink by 5 percent or
greater.
Lower House seats will be reapportioned from population-losing
prefectures to five mainly urban prefectures--Tokyo and its three
neighboring prefectures, as well as Aichi in central Japan.
Based on the preliminary census figures, 10 single-seat districts
will be added to five prefectures, with Tokyo receiving five more
seats, Kanagawa two more and one district each for Saitama,
Chiba and Aichi.
The 10 prefectures that would lose Lower House seats are Miyagi,
Fukushima, Niigata, Shiga, Wakayama, Okayama, Hiroshima,
Yamaguchi, Ehime and Nagasaki.
There would also be reapportionment in the proportional
representation constituencies, with the Tokyo bloc receiving
two additional seats and the Minami-Kanto bloc getting one
extra seat. The Tohoku, Hokuriku-Shinetsu and Chugoku
blocs would lose one seat each.
The actual reapportionment procedure will be handled
by a council under the internal affairs ministry and
the new district lines drawn up over the course of a year,
meaning the newly reapportioned seats will first be contested
in the Lower House election to be held after 2022.
Available electricity supply
June 24, 2021
“We hope that we will be able to ensure a stable supply of
electricity without causing any concern, especially due to
unplanned power outages and various problems,”
Kazuhiro Ikebe, Chairman of the Federation of Electric Power
Companies of Japan, said on June 18, 2021.
Under the country's electricity laws, utilities must maintain
a minimum reserve ratio of 3% of maximum demand as a safety
precaution against sudden shortages.
If the reserve ratio falls below 3%, there is risk of rolling
blackouts due to the inability to supply power when maximum
demand is reached.
Utilities generally secure a reserve ratio of 7% to 8% or more
to guard against a decrease in supply capacity due to power plant
troubles and increases in demand caused by high summertime
tempeatures.
At the end of May, eight of Japan's 10 major utilities predicted
their reserve ratios would be 3.7% in July and 3.8% in August,
when many people are expected to watch the Tokyo Olympics
and the Koshien high school baseball tournament from their
air-conditioned homes. Kyushu Electric Power Co. also predicted
a reserve ratio of 3.7% for July but it forecast that figure to rise
to 6.8% in August.
The reasons for the low forecasts, the Ministry of Economy, Trade
and Industry (METI) said in late May, was because of a decrease
in supply capacity caused by the suspension or closure of 10
older thermal power plants, including four operated by Tokyo
Electric Power Company Holdings Inc. (Tepco), two each
operated by Kansai Electric Power Co. (Kepco) and Tohoku
Electric Power Co., and one each belonging to Chubu Electric
Power Co. and Kyushu Electric.
The government predicted that summer electricity demand
would be at its most severe since 2017.
Russia's Summer Military Drills
Special News, June 23, 2021
Russia's Eastern Military District said Wedesday that
it has started large-scaled military drills involving
over 10,000 troops on the Kuril Islands, including isles
at the center of the country's territorial dispute with Japan.
Key military facilities of the Kuril Islands are located
on Etorofu and Kunashiri, two of the four Japanese-claimed
islands off Hokkaido. The drills are expected to be held
mainly on the two islands.
Some 500 military vehicles ,32 aircrafts and helicopters,
and 12 warships are set to participate in the drills.
Participants are seen practicing operations
to prevent Japan's landing,
attack targets with drones and deal with jamming.
Troops reportedly marched on Etorofu and Kunashiri for
more than 5 days.
Russia is increasing military facilities on the disputed
northwestern Pacific islands.
8月9日、スタ-リンのソ連軍、
突如、
満州に侵攻、
biglobe(火事泥強盗)始まる
1945年8月9日午前0時、戦車5250輌、航空機5171機からなる
ソ連軍が、当時、日本の関東軍が占領・支配していた満州国に
3方面から、一斉に侵攻してきた。その時、植田俊吉・関東軍司令官は、
大連で、観劇・宿泊しており、司令部所在の長春にはいなかった。
(注:謙吉とは別人)
戦争終了後の、極悪非道なスターリン・ソ連の
日本人捕虜奴隷労働虐殺犯罪と日本人女性強姦犯罪を
強く非難する
日本国民が正視しなければならない事実:
①日本政府が主張する「歴史的に日本の固有領土であったから
返還を要求する」は、ロシア政府にも、ロシア国民にも
100%完全に無視されている。まったく、相手にされていない。
②ロシア政府とロシア軍は、日本軍が攻めてくるという想定、
及び、ロシア軍が北海道に侵攻するという想定の下に
着実に、国後島と択捉島の軍事基地化を進めている。
③ロシア国民のほとんど全部は、日本人捕虜のシベリア虐待抑留
(=日本人捕虜の奴隷労働強制被害)をまったく知らず、
ロシアの北方四島領有は、国際社会に認知されている
正当なことであると、固く信じている。
日本人捕虜のシベリア虐待抑留(=日本人捕虜の奴隷労働
強制被害)についての罪悪感はまったくない。
日本外務省が隠蔽し
日本歴史から抹殺した
悲惨な日本人捕虜のシベリア虐待抑留
Re:
ソ連収容所における日本人捕虜の生活と死
この特別報告書は国立国会図書館及び外務省外交史料館所蔵の資料である。
掲載分については、平成18年7月20日、国立国会図書館の許可
(国図資050104005-6-12号・13号)を得てインターネットに掲載している。
原資料は米国国立公文書館に所蔵されている。
Abe's diplomacy with Russia to deceive Japanese people
July 16, 2017, Sunday
Abe & Putin has been continuing sparring over northern territories solution.
In the early morning of June 30, Hangul writing at a jetty construction site
jumped out at members of a Japanese public-private survey mission who had
arrived at Shikotan Island by ship to research joint economic activities between
Japan and Russia in the northern territories.
A South Korean company was joining the jetty construction.
“Russia was sending a message that if Japan didn't invest in the northern
territories, it could rely on South Korea, China, North Korea,etc.”
Abe and Putin agreed to begin discussing joint economic activities at a summit
meeting in December,2016. The idea is a pillar of Prime Minister Shinzo Abe's
“deceiving-Japanese people diplomacy.”
Announced in May 2016, the approach seeks to create an opening in the negotiations
over the territorial issue, which have made no progress in the more than 70 years
since World War II ended.
Possible candidate industries include tourism and fishing. However, since
Russia appears determined to proceed with the talks under the assumption that
any activities would be carried out under Russian law, creating a “special system”
that harms neither Japan's nor Russia's legal position will likely not be easy.
On July 6, one day before a Japan-Russia summit meeting in Hamburg, Germany,
Russian Deputy Prime Minister Yury Trutnev, who is in charge of development
in the Far East, jolted Japan by announcing that Russia would designate the northern
territories as a special economic zone of the Russian government.
Companies that expanded their activities there would receive tax benefits and
other preferential treatment, which would likely attract foreign businesses.
The move was seen as a “threat” that Russia would move forward
on development without Japan if the talks over joint economic activities dragged on.
The next day, Abe and Putin met for the 18th time. The special economic
zone was also discussed. Abe decisively told Putin the designation was “unacceptable”
because it could harm their plan for joint economic activities. Putin was evasive
in his response, and the exchange brought to light a difference between them.
Abe was enthusiastic as it could lead to a final settlement of the thorny issue
of postwar diplomacy. But when Putin visited Abe's home prefecture of Yamaguchi
in December,2016, for a summit meeting, Abe obtained nothing that would lead
to a return of the northern territories.
The international situation is thought to have been a major factor in this.
When Russia was isolated internationally after its annexation of the Crimea in 2014,
the Russian government is believed to have thought, “By engaging Japan, we can
poke a hole in the encirclement led by the United States.”
Things changed when Trump was elected U.S. president in November,2016.
Trump had shown a desire to improve relations with Russia, so “Putin's
enthusiasm toward Japan cooled.”
Re:
凄まじかった
鬼畜・ソ連軍兵士の強姦・残虐行為
ウィリアム・ニンモ氏の著書 『検証・シベリア抑留』 加藤隆訳 (時事通信社 1991年3月発行)
第47頁は
「要するに、満州・北朝鮮におけるソ連軍の日本人虐待は、
口ではいい表せないほどひどいものだった。
暴行と強奪は日常的だった。そして残虐な行為を犯した。
・・・とくに野獣のように乱暴なやり方で女を奪い、
抵抗するものは片っぱしから殺した。
ソ連軍の兵士たちが日本の女にしたことは、
いまでもぞっとするほど残虐なものだった」 と述べている。
強姦された日本人女性のほとんど総ては、
その後、自殺、絶望死、病死、衰弱死した。
【歴史の闇】に葬り去られた強姦被害
出典:『週刊朝日百科113 日本の歴史 現代③ 占領と講和』第79頁
凄まじいソ連軍兵士たちの強姦(レイプ)
現在は人口約700万人といわれ、ハルビン市、大連市と並ぶ満州
屈指の大都市、瀋陽市にソ連軍が侵攻してきたのは8月19日である。
すぐにハルビン市、長春市と全く同様にソ連軍兵士たちのレイプ
(強姦)凶暴・暴行・殺戮・略奪が始まった。
中国国防大学教官の徐焔(シュ・イェン)大佐は著書の
『1945年 満州進軍 日ソ戦と毛沢東の戦略』
(朱建栄(ツウ・ジェン・ロン)訳 三五館 1993年8月発行)
第223頁~第229頁からで次のように述べている。
「ソ連軍が満州に入った時点から、その相当数の将兵は直ちに、
横暴な行為を露骨に現した。彼らは敗戦した日本人に強奪と暴行を
振るっただけでなく、同盟国であるはずの中国の庶民に対しても
悪事をさんざん働いた。
特に強奪と婦女暴行の二つは満州の大衆に深い恐怖感を与えた。
100万以上の満州に出動したソ連軍兵士の中では、犯罪者は少数と
いうべきだが各地で残した悪影響は極めて深刻なものだった。」
満州でのソ連軍の軍紀の乱れは目に余るものがあった。
彼らは白昼堂々と倉庫の中のものを盗み出し、町で売りさばき、
得た金を着服した。
夜になると泥酔状態で臭気をまき散らしながら、町中「マダム」を
捜し回った。恐れおののく庶民はドアと窓を締め切り、
ソ連軍が一日も早く帰ることを内心に祈っていた。
満州の各大都市はどこも同じような状況で、夜になるとソ連軍兵士が
街角に現れ、通行人を止めては携帯物品を強奪し、女性を追い回し、
時には銃をもって民家に押しかけることもよくあった。
瀋陽の町ではソ連軍警備司令部の憲兵がトラックで巡行するのを
よく見かけた。酔っ払いと軍紀違反者が多すぎるため、トラックで
大量に収容するからだ。逮捕されたら厳しい処罰を受けるが、
それでも軍紀違反者が後を絶たない。
ソ連軍の軍紀退廃についての中国側の最初の報告は、
満州に進出した八路軍の一番手の部隊が延安の党中央に送った電報だ。
1945年9月初めに山海関を出て瀋陽に到着した部隊は、
ソ連軍兵士による強奪事件を目撃し、また多くの中国人市民から
訴えを受けた。その報告で、ソ連軍は「軍服はボロボロで、軍紀は
はなはだ悪い」と説明し、現地のソ連軍政治部にも
「軍紀を厳粛にせよ」と申し入れた。
ソ連軍政治部は「すでに多くの措置をとって軍紀違反者を罰しており、
多い日には一日に20人以上も処刑した」と回答した。
しかしソ連軍側はまた、その原因を、兵士のファシストに対する
敵愾心に帰し、ドイツでも同じ行動をしたと弁明した。
この回答に八路軍はもちろん満足することができない。かといって
それ以上どうしようもなかった。
ソ連軍は自ら非公式に次のように背景を説明した。ドイツとの
激しい戦争で大量の死傷者を出し、兵力補給の不足を来たし、
戦争後期、多くの刑事犯も軍隊に補給した。そのため軍紀の
引き締めが十分にできず、悪質者を一部銃殺して何とか規律を維持
しているという。この説明の内容は事実かも知れたいが、ソ連軍
首脳部が軍中の非行者とその行為を真剣に取り締まらず、事実上、
野放しにしたことの責任は逃れられない。
ファシストに対する敵愾心をもって兵士の非行を説明し、
中国を敗戦国のドイツに例えた譬えたことは、
八路軍の将兵の中で憤りを引き起こした。
仮に敗戦国だったにせよ、無辜な一般市民に狭量な民族報復を
働いていいということにはなるまい。
異国で「三日間勝手にせよ」として兵士の闘志を刺激するなど、
なおさら政治の堕落だ。
ソ連軍のこのようた釈明はまさに、大ロシア主義の態度を
反映したものだと言える。その根本的な原因はスターリンの
「共産主義総本山」の意識にあり、そのため他国の人民を尊重する
教育を怠ったのだろう。
ソ連軍の一個戦車軍団が1944年末にユーゴスラビアの片隅を
通過した。その短い道程で、千件以上の婦女暴行と強奪事件を
起こした。これがユーゴスラビア国民の強い反発を招き、
のちにユーゴとソ連の関係決裂になる原因の一つになった。
ソ連軍がドイッの東部を占領した後も、強奪と暴行を繰り返し、
ドイツ人の民族感情を傷つけた。本来は親ソ的な東ドイッ政権なのに、
統治の基盤が不安定だったのは、それが一因でもあった。
満州での行為は、ソ連軍の一貫した行為の東方での継続だ。
1969年4月の中国共産党第九回全国代表大会で
毛沢東がソ連の満州出兵に触れた際、
「当時のソ連の軍紀は退廃そのものだ」と恨めしげに語った。」
ソ連軍兵士の凄まじい婦女暴行②
若槻泰雄著 『戦後引揚げの記録 新版』時事通信社 1995年10月発行
第123頁
満州に侵入したソ連軍は、8月19日には、早くも外部との一切の通信交通を
遮断した。そして世界の目から隔絶された中で、ソ連の軍隊はほとんど例外なく、
被占領国民たる日本人の上に強奪・暴行・婦女暴行をほしいままにしたのである。
程度には若干の差はあったし、侵入直後が最も激しかった地区や、逆に日を追って
悪化したというような都市もあり、数日にして一応平静に帰した所もあれば、
占領の全期間、数ヵ月にわたった例もある。
兵器をもったソ連兵は、群れをなして日本人の各家庭や会社の事務所に押し入った。
そして手当たり次第、金めのもの時計、貴重品、衣類などを強奪する。
そして撫順など極めて少数の例外はあるが、婦人とみれば、老若を問わず
婦女暴行を働いた。
抵抗するもの、あるいは、これを阻止しようとするものは容赦なく射殺する。
窓を閉じ、扉に鍵をしめ、更には入口を釘で打ちつけていても無駄である。
軍隊が本気で民家に侵入しようとするならば、そんな程度のものを
打ちこわすのはいとも簡単であろう。家屋は無残にたたきこわされるだけだ。
しかもこの行動は「夜陰に乗じて」というわけではない。ソ連兵の強奪は
「盗む」とか、「奪う」というような段階ではなく、トラックを横付けにし、
「それはまるで運送屋のように、だれはばかることなく、せっせと運んだ」
と表現している体験記や、「何年もたった後でも、夜中エンジンの音を耳に
するとぞっとすることがあったくらいだ」という記述もある。
ホントは、イヒヒ?なんだよなぁ !! ワケワカラン・・・

Strive to create society
in which men,women
raise children together
June 20, 2021
The Child-rearing and Nursing Care Leave Law was revised
in the recent ordinary Diet session to make it easier for men
to take childcare leave.
A society must be created in which both men and women
are involved in raising children.
The rate of men taking childcare leave was still less than 8%
in fiscal 2019. This is a far cry from the government's
goal of 30% by 2025, and there is a clear gap between men and
women, with more than 80% of women taking childcare leave.
The situation in which the burden of child-rearing and
housework is disproportionately borne by women is
a major factor that discourages women from giving birth
and leads to a society with fewer children.
It also hinders women from continuing to work.
One of the pillars of the revised law is to improve
the workplace environment. The revised law obliges
companies to explain the details of the childcare leave
system to employees when they have a child, and
to confirm whether they intend to take such leave.
Companies need to actively encourage their employees
to take advantage of the system so that more male
employees will be involved in child-rearing.
Another pillar of the revisions is the creation of a new system
that allows men to take up to four weeks of leave within
eight weeks of the birth of their child.
Before the revision, child-rearing leave was in principle
to be taken once before the child turned 1 year old.
The introduction of the new system is said to make it easier
to split the leave taken between the immediate postnatal
period and again at a later time.
The revision is intended to al-low men to be involved in such
tasks as changing diapers, taking care of elder siblings and
doing housework at a time when women are under
a heavy physical and mental burden.
Russian President Vladimir Putin offered praise for former President Donald
Trump
in an interview with NBC News ahead of his June 16 bilateral summit meeting
with President Joe Biden.
Trump issued a statement on Thursday praising his relationship with Putin and
touting a 2018 meeting between the two in Helsinki, Finland. He also mockingly
offered
Biden good luck, saying "don't fall asleep during the meeting."
On Friday, Putin praised Trump and compared him favorably to Biden,
highlighting the president's long career in politics and saying his predecessor
was
an "extraordinary" person.
"Well even now, I believe that former U.S. president Mr. Trump is
an extraordinary
individual, talented individual, otherwise he would not have become U.S. president,"
the Russian president told NBC News' Keir Simmons.
"He is a colorful individual," Putin said. "You may like him or not.
And, but he didn't come from the U.S. establishment. He had not been part of big-time
politics before, and some like it, some don't like it but that is a fact."
Putin said Biden "is radically different from Trump because President
Biden is
a career man. He has spent virtually his entire adulthood in politics."
"That's a different kind of person, and it is my great hope that,
yes,
there are some advantages, some disadvantages, but there will not be any impulse-based
movements on behalf of the sitting U.S. president," he said.


Former Republican Representative Barbara Comstock urged members of her
party to stop
"fearing" former President Donald Trump and called on them to
support an investigation
into the events of January 6.
In an op-ed for The New York Times on Wednesday, Comstock called Trump
"the patron saint
of sore losers" and warned about unfounded claims of voter fraud in
the 2020 presidential election.
Comstock represented Virginia's 10th congressional district from 2015 to 2019. During the 2016
presidential election, she called for Trump to drop out of the race following
the release of
the Access Hollywood tape, which showed him using offensive language about
women.
Trump wants support for like-minded candidates
June 6, 2021, Sunday
Former U.S. President Donald Trump on Saturday
pushed Republicans to support those candidates
who share his values in next year's midterm
elections, as he launched a new more active phase
of his post-presidency.
The former Republican president teased the prospect
of another presidential bid of his own in 2024, but
vowed first to be an active presence on the campaign
trail for his allies in next year's fight for control of
Congress.
"The survival of America depends on our ability
to elect Republicans at every level starting with
the midterms next year," Trump charged.
Trump delivered his latest comments in a speech
to hundreds of Republican officials and activists
gathered for the North Carolina GOP convention,
the opening appearance in what is expected
to be a new phase of rallies and public events.
Out of office for more than four months and banned
from his preferred social media accounts, the former
president hopes to use such events to elevate his
diminished voice ahead of another potential presidential
run.
His advisers are already eyeing subsequent appearances
in Ohio, Florida, Alabama and Georgia to help bolster
midterm candidates and energize voters.


オレサマのカチカチヤマだな! あとは未曾有の七輪カジだが・・・・・ ニホンsaruもいるよ、吾韓撓!


Opposition to Tokyo Games grows
May 11, 2021,Tuesday
An opinion poll showed nearly 60% of people in Japan
want the Olympics cancelled less than three months
before they begin.
Prime Minister Suga said on Monday that he has never
"put the Olympics first.,"
The same day an opinion poll showed nearly 60% of
people in Japan want the Olympics cancelled
less than three months before they begin.
Japan has extended a state of emergency in Tokyo
until the end of May and is struggling to contain
a surge in COVID-19 cases, raising further
questions about whether the games should go on.
Its vaccination rate is the lowest among wealthy nations.
International Olympic officials, Tokyo planners and
Suga himself have insisted the games will go on
in "a safe and secure" way.
Foreign spectators have been barred and planners
issued an elaborate playbook of rules last month
aimed at preventing coronavirus infections.
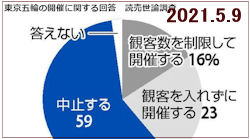
But a public opinion survey, conducted from Friday
through Sunday by the Yomiuri Shimbun daily,
showed 59% wanted the games cancelled as opposed
to 39% who said they should be held. "Postponement"
was not offered as an option.
Another poll conducted at the weekend by TBS News
found 65% wanted the games cancelled or postponed
again, with 37% voting to scrap the event altogether
and 28% calling for another delay.
More than 300,000 people have signed a petition
to cancel the games in roughly five days since it was aunched.
夢は何度でも!Mansee!!

延期による大会簡素化の議論が出た際、
手を付けられない“聖域”の存在が
あきらかになった。
2021.5.28
森喜朗前会長は「IOCには、
パーティーばかりやっている人たちが多数いるよ」と。
組織委は5月26日、延期前の18万人から7.8万人まで
大幅に人員削減した来日大会関係者についての内訳を公表した。
ゲストやスタッフは約6万人の削減に成功。そのほか、
国際連盟や放送関係者、プレスも削減した。
しかし、オリンピック・ファミリー3000人、各国
オリンピック委員会(NOC)1万4800人、パラリン
ピックファミリー2000人、各国パラリンピック委員会
(NPC)5900人の人数は、延期前の数字が維持されていた。
オリンピック・パラリンピック・ファミリーとは、
IOC役員、委員やIPC理事などの総称だ。
特に、IOC委員には王族や貴族が多く、開催都市選挙などでの
投票権をもっており、特権意識が強い。期間中、高級ホテルに
滞在し、IOCマネ-で、連日、豪華なパ-ティ-を繰り広げ、
オリンピックをエンジョイしていることから“互隣王貴賊”と
損承知されている。
組織委の森喜朗前会長は「パーティーばかりやっている
“五輪王貴族”には惨禍を遠慮してほしいのだが」と。
論議の結果、おもてなしの削減,各種セレモニーの中止、
ラウンジ、宴会サービスの縮小などを行うことにはなった。
ただ、来日する人数については、全員が、皆、訪日・歓待・
宴会を渇望しているので変更されなかった。
武藤事務総長は、「もともとこれらの人達は、IOC組織運営
のために必要不可欠な人材であることがほとんどなので、
現時点では代えることができない」と。
“互隣王貴賊”全員が、皆、訪日・歓待・宴会を渇望よ。


東京五輪中止提言、米紙から相次ぐ
2021.5.6
The U.S. papers propose
Tokyo Olympics cancellation
Washington Post, a influential newspaper proposes cancellation
for Japanese Government on 5th and published a column
to insist on when you should do "the loss cut" of the expense.
It is saying "the plunder should say to IOC that it be others
in Japan", and the column impeachs a posture of the IOC.
It made a cynical remark on Chairperson Bach with
"an overcharging baron".
Mention the Japanese public opinion which is negative
for holding, the stringency of the medical system,
and it "is irrational decision to host an international
mega event in a pandemic".
It published the article of the sports columnist "who should
not be held" about Tokyo Olympics while San Francisco
Chronicle was all parts of the world, and a spread of
the new coronavirus was prolonged on 4th.
Vaccination advances in the United States and can see
a sign to normalization, but does it when the situation
that is serious in India and European part, South
America continues,I claimed, "It run out of time"
for Tokyo Olympics holding.
Influential newspaper New York Times assumes it
"the worst timing" about the Tokyo Olympics holding
in the current situation in April and points out that
it may become "the big infection event" for Japan
and the world, with "the time when you should
reconsider the way of Olympics," It appeal.
Over 200,000 Signatures Collected
for Tokyo Games Cancellation
Tokyo, May 7, 2021, Jiji Press
For a petition calling for the cancellation of the Tokyo
Olympics and Paralympics, slated to be held this summer.
The petition, started at noon Wednesday by Kenji
Utsunomiya, 74, former president of the Japan Federation of
Bar Associations, claims that under the current circumstances "
it is certainly unlikely that the Tokyo Olympics could
be held safely."
"If the games are pursued, the Olympics would be denying
their very own purpose of 'celebrating peace,'" it argues.
The petition was launched on the Change.org website.
An English version has also been created.
The pace of gathering signatures for the petition is
the fastest since the website's Japanese version was
launched in 2012, according to its operator.
人、これ闘志、神(shin)でも天国でヤレ! Good had been helping me !! lahmen???

TENKASAN,KATARU【×騙る】
暦試勘慟物語り!鷺鵜はユメを実現!!なぁ?
 (日本某超有名収監試・秘報・日本の二人は、秘酔しても、オンリ-5億円!)
(日本某超有名収監試・秘報・日本の二人は、秘酔しても、オンリ-5億円!)
夢は何度も!
年収約19億円(鷺収入は無限・・・・・・嗚呼、韓驚・嵌涙・癇憤!)
比肩するものなき鷺世界一の天才ダモン!!

No ! No ! You, Left Devil !! ゆめはオワリ!!!
不忠・不義・不正・不服・不良・不届き・不信不満だらけの
バカタレケライドモめ が 革命・・・みな、ナコロでブッコロシたる!!!!!
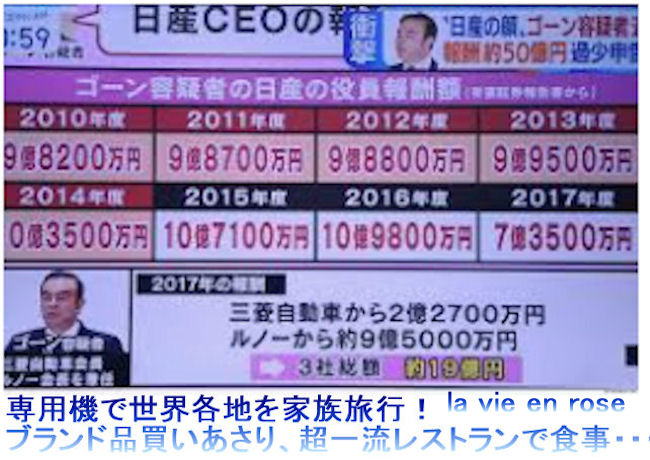
Nissan Chief Executive Makoto Uchida,
told a Japanese court on Wednesday,
July 7, 2021 that the company's former chairman,
Carlos Ghosn, had held too much power,
failed to listen to others, and stayed on for too long. 

むしょ震い止まらぬ仏蘭伯剌・摩湯の魔王鬼級の・・・
強怖い恐いオカタサマ!やるぞぅ!

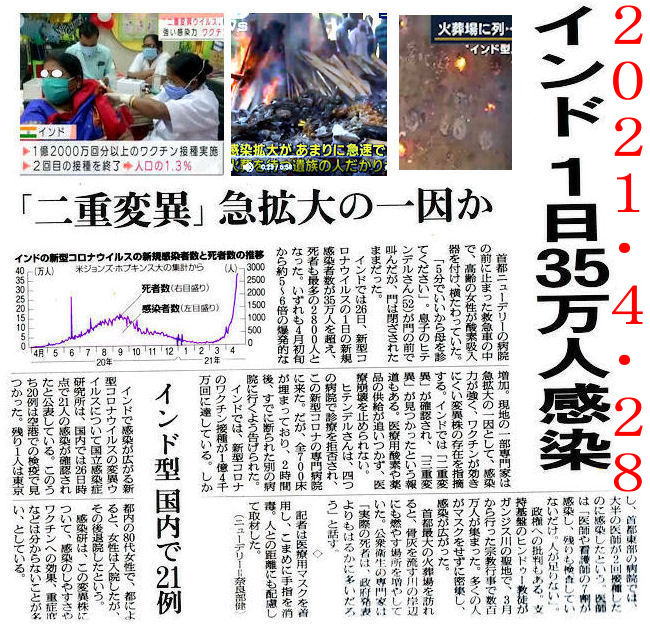
India Covid-19 numbers
May 2, 2021, Sunday

India Covid-19 numbers explained, finally, that breaks upward trend.
On May 1, Saturday, India's new cases of coronavirus
infections were 392,488 detected , about 9,500
less than the previous day's tally of 401,993.
This is the first time in more than a month that
the daily count of cases has been lower than
the previous day on a non-Monday.
For the entire month of April, the daily count of
cases increased on every single day, except
on Mondays, when the cases decline owing to
relatively lesser number of tests conducted
on a Sunday.
At the start of April, India had been reporting
less than one lakh cases a day. This rose to more
than 4 lakh cases a day, with the daily count
rising on every single day.

In India,there have been
record numbers of cases and deaths
May 2, 2021
Case numbers and deaths in India are continuing
to rise fast, fuelled by a new variant.
The country recorded the world's highest single-day
total on Thursday.
But the true numbers of cases and deaths are likely
to be higher than the numbers provided
by authorities, with many people avoiding testing or
struggling to access it. Many deaths in rural
areas also go unregistered.
Testing is increasing, but so too are the number
of positive results.
Last year, the World Health Organization recommended
countries needed to get the positive test rate below
5% for at least two weeks before considering easing
restrictions. The rate in India is now around 20%.
A high percentage of positive tests suggests high
infection rates and the likelihood of many more
people in the community with coronavirus undetected,
according to Johns Hopkins University.
In total, India has confirmed more than 18 million
infections and 200,000 deaths. Virologists say
they expect the rate of infections to continue
to increase for another two to three weeks.


India Coronavirus
Go Undercounted
April 25, 2021
India's coronavirus second wave is rapidly sliding
into a devastating crisis, with hospitals unbearably full,
oxygen supplies running low, desperate people dying
in line waiting to see doctors. And mounting evidence
that the actual death toll is far higher than officially
reported.
Each day, the government reports more than 300,000
new infections, a world record, and India is now seeing
more new infections than any other country by far,
almost half of all new cases in a global surge.
But experts say those numbers, however staggering,
represent just a fraction of the real reach of the virus's
spread, which has thrown this country into emergency mode.
Millions of people refuse to even step outside ?
Their fear of catching the virus is that extreme.
Accounts from around the country tell of the sick
being left to gasp for air as they wait at chaotic hospitals
that are running out of lifesaving oxygen.
The sudden surge in recent weeks, with an insidious
newer variant possibly playing a role, is casting increasing
doubt on India's official Covid-19 death toll of nearly
200,000, with more than 2,000 people dying every day.
Interviews from cremation grounds across the country,
where the fires never stop, portray an extensive
pattern of deaths far exceeding the official figures.
Nervous politicians and hospital administrators
may be undercounting or overlooking large numbers of
dead, analysts say.
And grieving families may be hiding Covid connections
as well, out of shame, adding to the confusion
in this enormous nation of 1.4 billion.
夢は何度でも!Mansee!!
'No Longer Purely Civil' by NY AG
Trump Org. Criminally Investigated, May 18, 2021
New York prosecutors have reportedly told
the Trump Organization that their criminal investigation
"is no longer purely civil."
Fabien Levy, press secretary to New York Attorney General
Letitia James, told CNN, "We have informed
the Trump Organization that our investigation
into the Organization is no longer purely civil in nature."
"We are now actively investigating the Trump Organization
in a criminal capacity, along with the Manhattan DA,"
he added. "We have no additional comment."
special collection
1日本-1945年:
大日本帝国陸海軍は1億総玉砕と叫び続けていたが
Japan in 1945
When the Emperor of Japan announced on August 15
in a radio broadcast-the first time the Japanese
people had heard the Emperor's actual voice - that
he was asking his people to "endure the unendurable,"
many who heard him did not know exactly what
he meant.
This was partially due to the static of the broadcast and
partly due to the formal language of the announcement.
A subsequent explanation of his words made
the message very clear:
the war was over and Japan had been defeated.
Japan had accepted the Potsdam Declaration
calling for "unconditional surrender."
But the Japanese people had already begun
"enduring the unendurable" in a different sense.
At the begining of 1945, the end-year of the Pacific War,
Japan was utterly devastated, with 2.1 million soldiers and
nearly 700,000 civilians dead, millions more ill and wounded.
Nine million citizens were homeless and the economy was
on the verge of collapse. Compared with the prewar
peak, only 40% of factories were able to operate at all.
Industrial output was around 10% of prewar levels.
With so many factories either destroyed or unable
to operate for lack of raw materials, over 2 million
workers were without jobs.
Those who were lucky enough to find work were
usually minimally paid.
With rampant inflation, whatever wages they were able
to earn bought progressively less in the marketplace.
Many citizens had escaped the sustained strategic bombing of
the major cities in the last year of the war in order to take
refuge in the countryside.
Tokyo's seven million residents in 1940 dwindled to three
million by war's end, and Osaka went from three million
to one million in the same time period.
Living standards in cities plummeted to 35% of prewar
levels, and to 65% in the countryside, where at least
some food could be grown.
It would be at least five years before the cities regained
their 1940 populations.
Japan had lost one-third of its total wealth and one-half of its
total potential income.
Inflation soared despite price controls on some foods
and commodities, making the black marketeers rich.
Military supplies disappeared into the hands of corrupt
officers, gangsters and industrialists at a time
when ordinary citizens were struggling for daily subsistence.

東京・浅草寺一帯:Photo by US Army
2ポツダム宣言
3生存かけて:飢え死は厭だ
4マッカ-サ-:恩人
5朝鮮戦争:まさしく神風
6講和条約:吉田茂
7高度経済成長:工業化大成功
8都市生活の変化:大学生大増加
9東京オリンピック:新幹線
①You Tube (2分30秒):リンカーンのゲティスバーグ演説
②You Tube(約9分):Roosevelt's Speech at the U.S. Congress
③You Tube(約14分): スティーブ・ジョブズ 2005スタンフォード大学卒業式演説


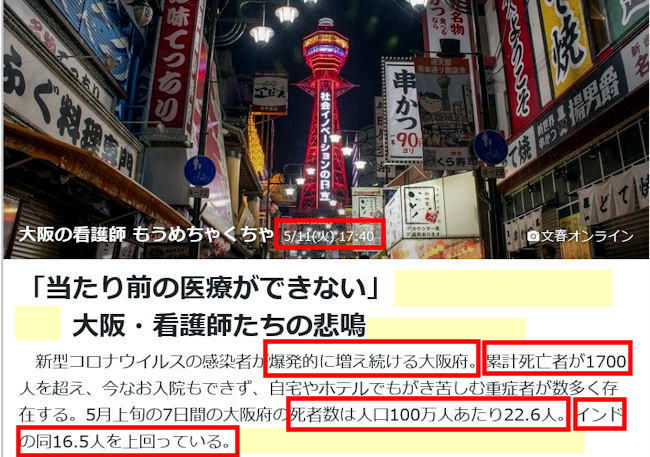
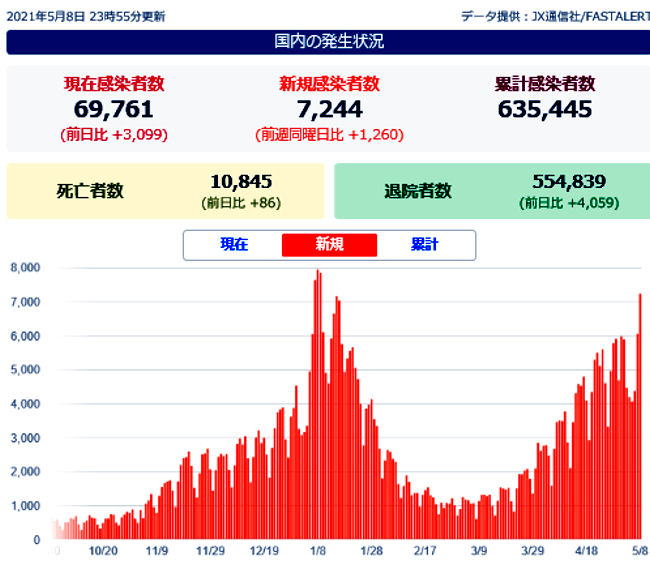
Japan's daily coronavirus cases top 7,244
May 08, 2021,Saturday
A total of 7,244 new cases of novel coronavirus
infection were confirmed across Japan on Saturday,
with the daily count topping 7,244 for the first time
since Jan. 16.
The nationwide number of severely ill coronavirus
patients stood at a record 1,131, unchanged from Friday.
The daily number of new COVID-19 cases hit record highs
in Aichi Prefecture, central Japan, at 575, and
Fukuoka Prefecture, southwestern Japan, at 519.
The two prefectures will be added on Wednesday
to the list of areas placed under the coronavirus
state of emergency.
New infection cases also hit record highs in 11
other prefectures.
Hokkaido, at 403, Okayama, at 189, Gunma, at 113,
Kumamoto, at 111, Oita, at 93, Ishikawa, at 80,
Kagawa, at 78, Saga, at 76, Shiga, at 74, Nagasaki, at 65,
and Niigata, at 50.
Tokyo confirmed 1,121 new infection cases, with its daily
total exceeding 1,100 for the first time since Jan. 22.
The seven-day average of new infection cases in Tokyo
came to 776.6, down 2.7 pct from a week before.
The number of COVID-19 patients with severe symptoms
under the Tokyo metropolitan government's standards
rose by two to 71.
Osaka Prefecture reported 1,021 new COVID-19 infection
cases, with its daily count falling below that in Tokyo
for the first time since March 29. New coronavirus
fatalities in Osaka came to 41, exceeding 40 for two days
in a row.


NEW DELHI May 6,2021,Thursday
India's devastating coronavirus crisis deepened
on Thursday as the country reported 412,000 infections
and nearly 4,000 deaths in the previous 24 hours.
Epidemiologists believe that India's surge could hit 500,000
cases a day in the coming weeks before retreating.
That would represent a ruinous burden for a health care
system already reeling from too many patients and
a shortage of crucial supplies like oxygen.
Last month, the United States advised its citizens
to leave India and on Thursday, the State Department
authorized the voluntary departure of non-emergency
personnel.
The era of massive change
May 3, 2021,Tuesday
It is important to go back to the Constitution,
the foundation of the nation, to tackle new issues.
Public debate must be deepened from a broad perspective.
The 74th Constitution Day has arrived. The basic
principles of popular sovereignty, respect for fundamental
human rights and pacifism have taken root among the people
and have become the foundation of post-war Japan.
It is our responsibility to protect these ideas and pass them
on to future generations.
However, the current state of the Constitution, which
has not been modified at all since its enactment, is
undesirable.
Many nations have revised articles in their constitutions
to reflect the changing times.
It is a matter of course for Japan to discuss amendments
if there are issues that need to be addressed.
Reality gap?
A new infectious disease has spread, threatening the lives
and livelihoods of the people.
Neighboring China has become a great military power,
heightening tensions in areas such as the East China Sea.
Information and communications technology is becoming
more sophisticated, while artificial intelligence is taking
over some of the work done by humans.
When the Constitution was enacted, it would have been
unimaginable that such an era would come. And it will be
difficult for a charter enacted more than 70 years ago
to adequately respond to an ever-changing Japanese society
and international situation.
Is there a gap between reality and the Constitution?
If the priority is simply to avoid revising the Constitution,
there is a risk that unreasonable interpretations will arise
and the "rule of law" will become a mere formality.
It is the responsibility of a legislature to review the articles of
a constitution, compile the desired changes into a proposed
revision and present the proposal to the people.



Stop drinking alcohol on streets
May 2, 2021,Sunday
As a measure to prevent the spread of the novel coronavirus,
the Tokyo metropolitan government has asked people
to stop drinking alcohol on the streets or in parks, and
has begun working with convenience stores to urge
customers to refrain from doing so.
At a Lawson outlet in Shibuya Ward, Tokyo, on Friday,
a message asking customers to stop drinking outdoors was
displayed on cash register screens when they bought alcohol.
The store also played in-store announcements asking
customers not to drink on the street or in parks.
The Tokyo metropolitan government, through an industry
group, has requested the cooperation of about 7,400
convenience stores in Tokyo.

Now ! second to A, B becomes to pass 400,000 COVID-19 deaths !

Blocked Suez container ship boarded
April 22, 2021, Thursday
Experts boarded the massive container ship Tuesday
that had blocked Egypt's vital Suez Canal and disrupted
global trade for nearly a week, seeking answers to
a single question that could have billions of dollars
in legal repercussions: What went wrong?
As convoys of ships again began traveling
through the artery linking the Mediterranean
and Red seas, a canal service provider said more
than 300 vessels carrying everything from crude
oil to cattle were still waiting for their turn
in a process that will take days.
Egyptian government officials, insurers, shippers
and others similarly waited for more details
about what caused the skyscraper-sized Ever Given
to become wedged across the canal on March 23.
When blame gets assigned, it will likely lead to
years of litigation to recoup the costs of repairing
the ship, fixing the canal and reimbursing those
who saw their cargo shipments disrupted.
Since the vessel is owned by a Japanese firm,
operated by a Taiwanese shipper, flagged in Panama
and now stuck in Egypt, matters quickly become
an international morass.

Smoky skies from the Northern California wildfires
Smoke from Northern California wildfires casts
a reddish aura in San Francisco
Just days before President Biden kicks off a climate summit
with world leaders, the United Nations World
Meteorological Organization released a report Monday
warning that “time is fast running out” to keep
global temperatures in check.
Titled “State of the Global Climate 2020,” the report
finds that concentrations of greenhouse gases
in the atmosphere continued to climb in 2020,
despite lockdowns imposed to slow the spread of
COVID-19.
Last year, the report notes, was the third warmest
on record, worsening the melting of glaciers and
sea ice, the acidification of the world's oceans and
the severity of wildfires and hurricanes.
A key goal of the Paris Agreement on climate change is
to keep global temperatures from rising above 1.5
degrees Celsius, and the U.N. report warns that
doing so will require a massive effort
from the governments of the world.
Osaka Logs Record 1,220
New Coronavirus Cases
April 19, 2021, Monday
Osaka Prefecture, western Japan, reported 1,220
new coronavirus cases on Sunday.
The daily count hit a record high for the prefecture
and exceeded 1,000 for the sixth straight day.
In Tokyo, the metropolitan government newly
confirmed 543 coronavirus cases on Sunday.
The daily tally in the Japanese capital topped 500
for the sixth consecutive day and was the highest
for any Sunday since the central government lifted
its second state of emergency over the virus
for Tokyo on March 21.
The number of severely ill people with the coronavirus
under the metropolitan government's criteria came to 45.
Winemaking replaces coal mining
in Sorachi region
April 18, 2021,Sunday
On a hill overlooking the Ishikari Plain at Tappu,
in the Hokkaido city of Mikasa, lies the Yamazaki
Winery, the leading winery in the Sorachi region.
Thirteen hectares of vineyards were covered
with white snow in early February, and the fragrant
smell of wine was emanating from the oak barrels
in the cellar.
"The grapes that were harvested in the fall are
maturing in the winter," said Taichi Yamazaki, 35,
who is in charge of viticulture and is also the winery's
manager.
Currently, the winery produces a total of 20 brands of
wine from 10 varieties of grapes, with a target of
producing 40,000 bottles a year.
The winery was established in 2002 by Yamazaki's
father, Kazuyuki, 68, a third-generation farmer.
The family planted European varieties, and after
four years of growing, they could finally harvest
the grapes.
The winery began making wine at its own facility.
The first variety they tried, pinot noir, was thought
to be difficult to grow in Japan. However, the grapes
that grew on the slopes of Tappu, exposed to dry
southerly winds, soon gained recognition both in
Japan and abroad.
In 2008, Robert Parker, arguably the world's most
influential wine critic, gave Yamazaki's wine the
highest score in Japan at the time.
"That made us overcome a hurdle that we had to
overcome," Yamazaki said.
In 2019 and 2020, the Yamazaki Winery successively
received the highest five-star rating at the Japan
Winery Awards, which recognize fine wineries in Japan.
Yamazaki recalls, "I wasn't interested in the awards
race, but I was happy that the local people were pleased
to hear Mikasa has something to be proud of."
Following the lead of Yamazaki and his family,
the number of wineries in the Sorachi region, which
was only two in 2008, has now increased to six,
making Sorachi one of the leading wine-producing
regions in Hokkaido.
Yamazaki now hopes to develop a wine industry that
is closely connected to the region. The quality and
name value of locally grown grapes play a major role in
the value of wine. The Sorachi region, including
Mikasa, has many favorable qualities as a wine-
growing region and can strongly appeal to both domestic
and foreign markets.
The wine industry is also linked to the region's agriculture,
tourism and education. Yamazaki serves as a
school councilor for the municipal Mikasa High School,
which is known for operating Hokkaido's first "high
school restaurant," where students do the cooking and
table waiting. He advises the school on its management.
The municipalities in Sorachi, in a former coalproducing
region, have suffered demographic and economic decline
since the closure of the mines.
In recent years, more and more people have been coming
to Mikasa for the wine, and mail order sales have been
increasing amid the coronavirus pandemic.
Yamazaki said he has a far-reaching plan to "make wine
an alternative industry to coal mining."
Booklet published to introduce local wineries
The Hokkaido Government Sorachi General Sub-prefectural
Bureau has published the booklet "Sorachi Wine Guide,"
which highlights wineries and farms in the region.
The booklet introduces the history of wine in Sorachi,
depicting six wineries and nine farms with photos,
notes and maps.
The guide also includes information on local specialties
that can be enjoyed with wine, hot springs and hotels
in the region, and stores in Sapporo and other
areas where Sorachi wine can be purchased.
Record highs in Osaka, Hyogo
April 17, 2021, Saturday
Osaka Prefecture logged a record 1,209 new coronavirus
cases on Friday, marking the fourth consecutive day
that the daily figure has exceeded 1,000.
Hyogo Prefecture also announced its highest daily tally of
510 cases.
Meanwhile, Tokyo confirmed 667 new cases, marking
the 16th consecutive day thedaily figure has exceeded
the number from the same day in the previous week.
The number of seriously ill COV I 1)-19 patients
in the capital increased by six to 43.
The average number of neiv infections in Tokyo over
the past seven days was 542, up by 22.9%
from the previous week's average of 440.9,
according to the Tokyo metropolitan government.
The spread of highly infectious coronavirus variants
across Japan has put the government on high alert.
At a panel meeting held on the same day, Yasutoshi
Nishimura, minister in charge of economic revitalization
and the nation's pandemic response, said,
"It is estimated that variants will come to almost
completely replace the original virus in the Tokyo
metropolitan area, the Kansai region, and the Chukyo
region in May. We must respond with the utmost vigilance."
An uptick in variants has been observed in Aichi Prefecture.
The number of new infections with variants has also been
on the rise in Saitama, Chiba and Kanagawa, especially
in areas close to the border with Tokyo, where priority
measures have already been applied.
Priority measures went into effect in Osaka, Hyogo
and Miyagi prefectures on April 5, followed by Tokyo,
Kyoto and Okinawa prefectures on April 12.
Japan Logs Over 4,000 New
Coronavirus Cases for 3rd Day
April 16, 2012,Friday
The daily number of new coronavirus cases in Japan
came to 4,532 on Friday, surpassing the 4,000 mark
for the third consecutive day.
The western prefecture of Osaka reported 1,209 new
coronavirus cases on the day, rewriting its daily record
high and marking its fourth straight day with more
than 1,000 new cases.
The daily count of new cases also hit a record high
in the neighboring prefecture of Hyogo, at 510, and
the central prefectures of Niigata and Ishikawa,
at 40 and 35, respectively.
Across the country, 46 new deaths were reported
among infected people, including 16 in Osaka.
The number of infected people with severe symptoms
stood at 670, up 39 from the previous day.
In Osaka, the occupancy rate for hospital beds
secured for severely ill patients with the coronavirus
reached 102.2 pct, as the number of such patients
climbed to yet another record high of 274.
Skillful Matsuyama makes history
with Masters victory at Augusta
April 15, 2021
It can be said that his efforts to improve his skills
by competing with rivals on difficult courses overseas
have come to fruition.
The achievement of a long-cherished dream
in Japanese golf must be applauded and the joy
must be shared.
Hideki Matsuyama won the Masters Tournament,
a major men's golf tournament.
He is the first Japanese man to win a major.
In an interview after his victory, Matsuyama reflected
on the tough battle, saying, "All I could think
about was doing my best."
On the third day, he was alone at the top
with a four-shot lead over second place.
But he struggled on the last day, hitting his second shot
into the water at a crucial moment in the final stages
as the pressure mounted.
Many people must have been moved by the fact that
he was able to endure the breathtaking tension and
grab the victory.
The majors are the most prestigious golf tournaments
in the world. The Masters, in particular, is a stage
where the world's best golfers gather, and the venue,
Augusta National Golf Club, is known as a great course
with many difficult holes to conquer.
Expedite efforts to secure personnel
for campaign to vaccinate elderly
April 14, 2012, Tuseday
Vaccines are the decisive factor in containing the novel
coronavirus pandemic.
First of all, efforts to secure medical workers must be
expedited to enable prompt vaccinations of the elderly,
who are more likely to become seriously ill.
An initial batch of doses for about 50,000 people was
distributed for the coronavirus vaccinations
for the elderly that started on Monday.
The vaccine is said to have been administered
in most prefectures.
The vaccine being used is manufactured by Pfizer Inc. of
the United States. Two doses have been shown to be
95% effective in preventing the onset of COVID-19.
In the United States, it has been reported that
infections and deaths in elderly care facilities have been
reduced by more than 90%.
The vaccination rollout must be expedited in Japan.
According to a survey of medical workers who received
priority vaccinations in Japan, those aged 65 and older
were less likely to experience side effects than younger
generations.
However, people should be prepared for side effects
such as fever, headache and lethargy.
Although there is no need to be overly concerned
about side effects,there may be many elderly people
who find it difficult to communicate clearly.
It is essential for medical workers and facility staff
to carefully observe those who have been vaccinated
and take all possible measures to manage their physical
condition.
Osaka's Daily Coronavirus Tally
Tops 1,099 for First Time
April 13, 2021,Tuesday
The Osaka prefectural government said Tuesday that
it has confirmed more than 1,000 new cases of novel
coronavirus infection the same day, a first for
the western Japan prefecture.
The daily tally in Osaka stood at 1,099, beating
the previous record of 918 marked on Saturday.
Meanwhile, neighboring Hyogo Prefecture reported
a record 391 infection cases on Tuesday.
Tokyo logged 510 new infections, the first reading
above 500 in three days, and the southern prefecture of
Okinawa confirmed 125 new cases, the first figure
above 100 in three days.
In Tokyo and Okinawa, pre-emergency measures
against the pandemic started on Monday.
The daily count of new cases in Aichi Prefecture,
central Japan, came to 168, exceeding 100 for
the eighth straight day.
Osaka Prefecture recorded 1,099 new cases of infection
with the novel coronavirus on Tuesday, the highest daily
tally seen in the prefecture, sources close to
the prefectural government have said.
It was the first time that the daily number exceeded 1,000
in the prefecture, surpassing the previous high of
918 set on Saturday.
In Tokyo, 510 people were newly confirmed as having
contracted the virus, the first time in three days that
the daily figure was above 500.
It was also the first time for the capital to surpass
500 on a Tuesday since the 556 cases logged on Feb. 2.
The seven-day moving average of new cases per day
was 492, an increase of 24% from the previous seven-day
period of 396.9 cases.
Variants spreading
rapidly in Kansai
Friday, April 9, 2021
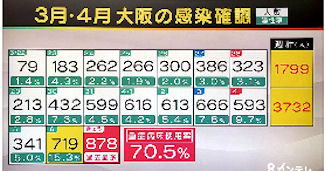
Osaka virus cases top 905
Thursday saw 905 newly confirmed cases of infection
with the novel coronavirus in Osaka Prefecture,
according to the prefectural government.
The prefecture reached a record high for daily infections
for the third day in a row.
Mentation of priority measures is limited to certain
municipalities or areas, such as entertainment districts.
The Tokyo metropolitan government said Thursday
that 545 new cases of novel coronavirus infections
had been confirmed in the capital, exceeding 500
for the second day in a row.


Fears of '4th wave' rise with surging
rural Japan coronavirus infections
March 27, 2021
Drastic increases in coronavirus cases are being witnessed not just
in highly populated areas like Tokyo and Osaka, but also in Japan's
regional areas, in a trend prompting experts to admit concern that
a "fourth wave" of infections may be coming.
The coronavirus cases resurgence has produced alarm among experts,
while at the same time a series of infections by variant strains thought
to be highly transmissible have also been confirmed across the country.
Japan Medical Association President Dr. Toshio Nakagawa said,
"Infection tallies in Tokyo have been surpassing earlier weeks'
for consecutive days," and indicated at a March 24 press conference
that he feels a sense of crisis over the latest state of infections.


Blowups With China and Russia
Saturday, March 20, 2021
It may look like the bad old days of the Cold War,
but today's bitter superpower competition is about
technology, cyberconflict and influence operations.
Secretary of State Antony J. Blinken and
the national security adviser, Jake Sullivan,
on Friday in Anchorage, where they met with their
Chinese counterparts for two days of rocky talks.
Sixty days into his administration, President Biden got
a taste this week of what the next four years may
look like: a new era of bitter superpower competition,
marked by perhaps the worst relationship Washington
has had with Russia since the fall of the Berlin Wall,
and with China since it opened diplomatic relations
with the United States.
It has been brewing for years, as President Vladimir
V. Putin of Russia and President Xi Jinping of China
took sharp turns toward authoritarianism.
But it blew up in open fashion this week, after
Mr. Biden agreed with the proposition that Mr. Putin is
a “killer” and the Chinese, meeting with the United
States for the first time since the new administration
took office, lectured Americans about the error of
their arrogant view that the world wants to replicate
their freedoms.
A lot of it was for show, on both sides, with cameras
whirring. All of the participants were playing to their
domestic audiences, the Biden team included.
But it was not entirely an act.
While the Cold War has not resumed — there is
little of the nuclear menace of that era, and
the current competition is over technology,
cyberconflict and influence operations — the scenes
playing out now have echoes of the bad old days.
As a moment in theatrical diplomacy, the meeting
on Thursday and Friday in Anchorage between
the Americans and Chinese was reminiscent of
when the Soviet premier, Nikita S. Khrushchev,
made headlines around the world 60 years ago
by banging his shoe on a desk of the United Nations
and shouting about American imperialists.
But as veterans of the old Cold War will suggest,
the superpower rivalries today bear little resemblance
to the past.
Mr. Putin himself has lamented that the Russia of
the early 21st century is a shadow of the Soviet
Union that trained him to be a K.G.B. agent.
Russia’s economy is roughly the size of Italy's.
Its greatest power now is to disrupt and instill fear,
using nerve agents like Novichok to silence dissenters
around the world, or deploying its cyberability to bore
deeply into the networks that keep the United States
humming.








All genetic result!
Floyd family agrees to
$27 mil. settlement
Sunday, March 14, 2021
The city of Minneapolis on Friday agreed to pay
$27 million to settle a civil lawsuit from George
Floyd's family over the Black man's death in police
custody, as jury selection continued in a former
officer's murder trial.
Council members met privately to discuss
the settlement, then returned to public session
for a unanimous vote in support of the massive
payout.
It easily surpassed the $20 million the city approved
two years ago to the family of a white woman killed
by a police officer.
Floyd family attorney Ben Crump called it the largest
pretrial settlement ever for a civil rights claim, and
thanked city leaders for "showing you care about
George Floyd."
"It's going to be a long journey to justice.
This is just one step on thejourney to justice,"
Crump said. "This makes a statement that George Floyd
deserved better than what we witnessed on May 25,
2020, that George Floyd's life mattered, and that
by extension, Black lives matter."
"Even though my brother is not here, he's here
with me in my heart," Philonise Floyd said.
"If I could get him back, I would give all this back."
The settlement includes $500,000for the south
Minneapolis neighborhood that includes the 38th
and Chicago intersection that has been blocked
by barricades since his death, with a massive metal
sculpture andmurals in his honor.
The city didn immediately say how that mone
would be spent. Floyd was declared dead on March 25.
Overnight hotel stays
down by half in 2020
Sunday, Feb.28, 2021
The total number of nights that guests stayed at hotels
and ryokan (Japanese-style inns) in Japan in 2020
plunged 48.9% from the previous year to 304.8 million,
preliminary data showed Friday.
Voluntary restrictions on travel and tougher border controls
amid the novel coronavirus crisis caused the slump.
Japanese travelers accounted for 286.77 million, down 40.3%,
while foreign travelers numbered 18.03 million, down 84.4%,
according to the data, released by the Japan Tourism Agency.
Both numbers sank to the lowest levels since the current
survey method was adopted in 2010.
Among the country's 47 prefectures, Osaka saw the biggest
drop at 63.9%, followed by 62.3% for Tokyo and 61.1%
for Okinawa Prefecture.
Across Japan, the total number of nights stayed by guests
plunged more than 80% year on year in April and May
last year,when the country's first coronavirus state of
emergency was in place.
The size of the drop narrowed to 30% to 40% between
September and December thanks to the government's
Go To Travel tourism promotion program.
The average hotel and ryokan room occupancy rate in 2020
hit a record low of 34.6%, compared with around 50%
to 70% in usual years.
Last month, the total number of nights stayed by guests
plummeted 61% from a year before to 16.81 million.


大寒波に凍てつく米国テキサス州の最南端にある、いつもは爽やかな海辺の町
サウス・パドリー・アイランドの大部分でも、電気・水道が止まり、携帯電話の
電波も不安定だ。
No-money-monkeys-calling 'Let's escape from here'


A rare deep freeze forced primates
at a large animal sanctuary in Texas
Thursday, Feb.18, 2021
So many animals have died after suffering a power outage
amid the historically cold weather that has slammed
the state and left millions without electricity.
Primarily Primates – a non-profit animal sanctuary that
provides care for hundreds of animals in Bexar County,
Texas – has not had power since Monday and was
among the over four million customers left without power
after a rare deep freeze forced the state's electric
grid operator to impose rotating blackouts across the region.
Newborn giraffe dies when mother accidentally steps
on its neck after giving birth.
“While the staff and volunteers work tirelessly around
the clock to evacuate dozens of animals from
the 78-acre sanctuary and use heaters and generators
to keep the remaining animals safe—they are also
mourning the loss of approximately many animals,
including monkeys, lemurs and one chimpanzee,”
the sanctuary said in a statement on Wednesday.
As of 10:30 p.m. on Wednesday night, just under
1.9 million customers in Texas are still without power
due to the inclement weather.
"This is the winter version of Hurricane Harvey,"
Texas Gov. Greg Abbott told Houston ABC station KTRK.
"And we will learn from this also, and we will come up
with strategies to make sure there are available sources
of power and energy so that things like this do not
happen again."
The Electric Reliability Council of Texas (ERCOT),
the agency that oversees the state's grid, entered
its highest alert level overnight into Monday and
ERCOT asked residents to close their blinds,
unplug unused appliances, postpone doing laundry,
wear warmer clothes and set thermostats no higher
than 68 degrees.
この氷点下で、大勢の住民が,徒歩とボートで生き物を救助している。
この島の名物で、絶滅の危機に瀕してもいる、ウミガメたちだ。
2月16日夜までに、地元の保護団体「シータートル」のボランティアは、
昏睡状態のカメ3,500匹以上をリハビリのため、
町のコンベンションセンターに運んだ。

People saved hundreds of sea turtles
Thursday, Feb. 17. 2021
The deadly winter storm that swept across Texas and
parts of the South knocked out power and water
for millions.
It also created a catastrophe for animals statewide —
including for sea turtles prone to freezing in frigid waters.
Bellamy, an Army and Marine Corps veteran spotted
some turtles Tuesday with his son Jerome.
But he needed help. He alerted Capt. Christopher
Jason, the commander of Naval Air Station Corpus
Christi in southeastern Texas, and his wife,
Cheryl Jason. The commander grabbed his kayak,
paddled into the cold waves and retrieved
a lapful of cold-shocked turtles.
But the next day, on Bellamy's turtle patrol,
the situation became far more urgent, he said,
and one that would require a lot more hands.
“It was like an apocalypse of turtles littered
on the beach,” Bellamy told The Washington Post
in a phone interview Thursday.
More than 1,100 turtles have since been plucked
from Laguna Madre by a ragtag group of about 50 Navy
pilots and flight students, military spouses, family
members and military retirees, said Biji Pandisseril,
the Navy installation’s environmental manager.
More turtles are still coming in, he said, and some have died.
Green sea turtles, listed as a threatened species,
feast on grasses found in the waters of Laguna Madre,
but in winter weather, the chilling shallow water
zaps strength from the coldblooded reptiles.
They become immobile and unable to power their fins
to warmer, deeper waters, putting them at risk of
dying of predation or exposure, according to
the National Park Service. Some wash ashore like driftwood.
Rescuing “cold-stunned” turtles has become an annual
routine in Texas, with dozens or hundreds aided
in a typical year, a spokeswoman for the conservation
group Sea Turtle Inc. told The Post.

Nikkei tops 29,000
for 1st time in over 30 years
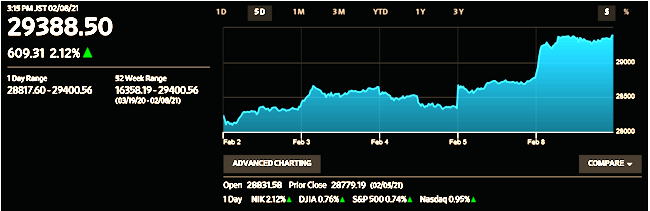
Tuesday,Feb.9, 2021
The benchmark Nikkei stock average closed above
29,000 for the first time in 30 and a half years on Monday,
lifted by a continued Wall Street rally on hopes
for a large-scale stimulus package.
The Nikkei average of 225 selected issues listed
on the First Section of the Tokyo Stock Exchange
soared 609.31 points, or 2.12%, to close at 29,388.50,
the highest finish since Aug: 3, 1990.
On Friday, the key market gauge jumped 437.24 points.
The TOPIX index of all TSE First Section issues finished
up 33.00 points, or 1.75%, at 1,923.95, a level unseen
since June 5, 1991. The index gained 25.83 points
the previous trading day.
Tokyo stocks spurted right after the opening bell
on broad-based buying spurred by all three major
U.S. market indicators, including the Dow Jones
industrial average, extending their rising streaks on Friday.
Buying sentiment was boosted by the approval of
a budget resolution by both the U.S. House and Senate
that enables President Joe Biden's $1.9 trillion coronavirus
relief package to passed through Congress
despite Republican opposition.
"Buying was led by players with short positions,"
a midsize brokerage house official said.
After the initial upsurge, however, the market became
top-heavy due to stepped-up selling to lock in gains.
On the TSE First Section, gainers overwhelmed decliners
1,710 to 429, while 52 issues were unchanged.
Volume inched up to 1.585 billion shares from Friday's
1.537 billion shares.
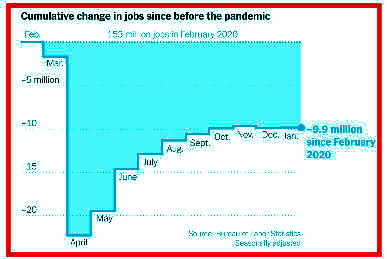
Anemic(貧血症で無気力な) Jobs Report
Reaffirms Pandemic's Grip
on Economy
Feb.6, 2021
With few of those in the private sector, the labor market
offers little relief to the nearly 10 million Americans
who are unemployed.
The American economic recovery showed new signs of
stalling on Friday as government data underscored
the pandemic's brutal damage to the job market.
U.S.employers added 49,000 jobs in January,
the Labor Department said, dashing hopes that
the new year would bring immediate relief.
The private sector added just 6,000 jobs,
barely enough to register against the millions of
positions lost during the pandemic.
The weak showing was tallied amid a fresh effort
in Washington to provide a big infusion of aid to foster
a recovery and the data will almost certainly bolster
the Democrats' argument for a robust stimulus package.
“It's very clear our economy is still in trouble,”
President Biden said of the latest reading
on the labor market.
Looking to strengthen the recovery,
Mr. Biden and congressional Democrats have been
pressing for a $1.9 trillion relief measure.
By approving budget resolutions in both chambers,
Congress cleared the way on Friday to pursue
final passage of the package on party-line votes,
if needed, within weeks.
Some Republicans have asserted that a smaller
package would suffice, and others have said it is
too soon for another round of aid.
But far from showing a job market on the mend,
the report on Friday provided evidence of
a metastasizing crisis.
The limited January gains followed an outright
setback in December, when the economy
lost 227,000 jobs, the first net decline since April
and a bigger drop than had been initially reported.
And while the December losses were concentrated
in a few pandemic-exposed sectors, the weakness
in January was broad-based.
Manufacturers, retailers and transportation
companies all cut jobs, indicating that
the economic damage is spreading.

Sony got a lift from the popularity
Feb.5, 2021
Sony's fiscal third quarter profit jumped 62 percent,
positioning the Japanese entertainment and electronics
giant for a record annual profit as its bottom line
got a healthy boost from its mega-hit animation film
“Demon Slayer.”
Tokyo-based Sony reported Wednesday a 371.9 billion yen
($3.5 billion) profit for the October-December period,
up from 229.5 billion yen a year earlier.
Sony's video-games sector thrived as people stuck
at home for the coronavirus pandemic turned
to such entertainment content.
Sony's quarterly sales edged up 9 percent to 2.7
trillion yen ($25.7 billion).
Sony also got a lift from the popularity of its Play-
Station 5 console, which went on sale late last year,
and did well in software and online-service sales,
despite launch costs, the company said.

Shiseido to Sell Personal Care Biz for 160 B. Yen
Tokyo, Feb. 3, 2021, Japanese cosmetics maker Shiseido Co. said Wednesday
that it will sell its personal care goods operations to European investment
fund
CVC Capital Partners for 160 billion yen.
The operations include the "Tsubaki" hair care product brand
and
the "uno" hair and skin care product brand for men
The business, which has well-known product brands
such as Tsubaki, will be transferred on July 1
to a company to be set up by the CVC fund in the
first half of this year, Shiseido said.
The beauty giant said it will then acquire a 35 percent
stake in the new company and continue to engage
in the business, which made up about 10 percent of
its total sales in the business year ended December
2019. The segment raked in sales of over 100 billion
yen in the period.
"Although we have hit products such as Tsubaki,
we faced the intense dilemma that we could not
work out our priorities," Shiseido President Masahiko
Uotani said at an online press conference, when
he explained the reason for the sale of
the personal care business.
Given that there were many other rivals in the sector of
personal care items and the rate of profitability was not
that high, increased investment for product development
and advertising was weighing on its bottom line.
Shiseido's sales, supported in recent years by a massive
inflow of foreign tourists to Japan, have been hit
by the coronavirus pandemic.
Shiseido, which projects a net loss of 12 billion yen
for the business year through Dec. 31, will report
its full-year earnings next Tuesday.

Scaled-down setsubun ceremony
held in Naritasan
Feb.3, 2021
A scaled-down setsubun ceremony was held at Naritasan
Shinshoji temple in Chiba Prefecture, on Tuesday,
marking the day before the beginning of spring according
to the lunisolar calendar.
Sumo wrestlers and actors usually participate in the annual
event at the temple, throwing dried soybeans into the crowd
to drive away evil spirits and bring good luck.
However, no such famous figures were present this year,
as the event was scaled back due to the coronavirus crisis.
Instead, men who were born in the Year of the Ox,
this year's Chinese zodiac I sign, threw out the beans.
This was the first time since 1969 --- when the event
at the temple took its current form -- that celebrities
did not participate.
The venue in front of the temple's main hall, which
is usually crowded with 50,000 to 60,000 worshipers
in ordinary years, was sparsely populated.
Job openings ratio
marks largest drop in 45 yrs
Saturday, Jan.30, 2021
The average ratio of effective job openings to job
seekers in the nation in 2020 fell 0.42 points
from the previous year to 1.18, marking
the largest drop since 1975, or right after
the first oil crisis, the labor ministry said Friday.
While job openings decreased by 21%, reflecting
sluggish corporate earnings amid the coronavirus
pandemic, job seekers increased by 6.9% as many
people were dismissed or saw their job contracts
terminated, according to officials of the Health,
Labor and Welfare Ministry.
The decline of the job openings ratio, representing
the number of jobs available to each of the applicants
registered at HelloWork job placement offices across
the country, was steeper than a 0.41-point drop
marked in 2009 in the aftermath of a financial
crisis triggered by the collapse of Leh-man Brothers.
In 1975, the ratio slid 0.59 points.
In 2020, the ratio deteriorated especially
in the accommodation and restaurant services sector,
which was hit hard by the government's first
declaration of a state of emergency over the coronavirus
epidemic in spring 2020 and people's voluntary
restrictions on going out amid the virus crisis.
Separately, the Internal Affairs and Communications
Ministry said the average unemployment rate in 2020
rose by 0.4 percentage points from the previous year
to 2.8%, up for the first time in 11 years.
The number of jobless people grew by 290,000 to 1.91
million, also marking the first rise in 11 years.
The number of workers on temporary leave came
to 2.56 million, hitting the highest level since
comparable data became available in 1968.
In December last year, the ratio ofeffective job
openings to job seekers stood at 1.06, unchanged
from November.
The seasonally adjusted unemployment rate stood
at 2.9% in December, also unchanged from
the previous month.
Uncertainty is widely seen to be looming over
the future employment situation in Japan after
the government declared a fresh state of
emergency over the epidemic earlier this month.
"The situation is expected to become tougher,"
Health, Labor and Welfare Minister Norihisa Tamura
told a news conference on Friday.
 Samsung chief Lee Jae-yong
2021.1.26
Samsung chief Lee Jae-yong
2021.1.26
Samsung chief failed to appeal his two-and-a-half-year jail sentence
for bribing ex-President Park Geun-hye by the deadline Monday."Lee
has humbly accepted
the court's judgment and decided not to appeal," his lawyer said.
It seems Lee concluded any appeal would be pointless since the Supreme
Court
already back the case to the Seoul High Court in August 2019.
In a press release, prosecutors said they respects the Seoul High Court's judgment
on Jan. 18, saying their "goal of finding the truth in the corruption
and bribery scandal
that brought down Park Geun-hye has been achieved."
Since Lee already served about a year on remand, he has to stay in jail
until July 2022 unless he is released early.
 Merck & Co.
Merck & Co.
ends COVID vaccine program,
cites inferior immune responses
January 25, 2021
Drugmaker Merck & Co. said on Monday it would stop
development of its two COVID-19 vaccines and focus
pandemic research on treatments, with initial data on an
experimental oral antiviral expected by the end of March.
Merck was late to join the race to develop a vaccine
to protect against the coronavirus, which has so far
killed more than 2 million people and continues to surge
in many parts of the world including the United States.
The company will record a pre-tax discontinuation charge
in the fourth quarter for vaccine candidate V591, which
it acquired with the purchase of Austrian vaccine maker
Themis Bioscience, and V590, developed with nonprofit
research organization IAVI, Merck said in a statement.
In early trials, both vaccines generated immune responses
that were inferior to those seen in people who had
recovered from COVID-19 as well as those reported
for other COVID-19 vaccines, the company said.
The announcement is a setback to the fight against
the pandemic and comes a month after Sanofi and
Glaxo Smith Kline delayed launch of their shot to late
2021, underscoring the challenges of developing vaccines
at record speed.
Tens of millions of doses of vaccines from rivals Pfizer Inc
and German partner BioNTech as well as from Moderna Inc
have so far been administered globally.
Johnson & Johnson, AstraZeneca Plc and others are also
racing to develop safe and effective vaccines to protect
against the virus.
Merck said it would focus COVID-19 research and
manufacturing efforts on two investigational medicines:
MK-7110 and MK-4482, which it now calls molnupiravir.
Molnupiravir, which is being developed in collaboration
with Ridgeback Bio, is an oral antiviral being studied
in both hospital and outpatient settings.
Merck said a Phase 2/3 trial of the drug was set to finish
in May, but initial efficacy results were due in the first quarter
and would be made public if clinically meaningful.
Merck said results from a Phase 3 study of MK-7110,
an immune modulator being studied as a treatment
for patients hospitalized with severe COVID-19, were
expected in the first quarter.
Shares of Merck fell 1% to $80.12 in trading before the bell.

Chinese fighter
ramped up its pressure
on democratic Taiwan
Jan.25, 2021
Large number of fighter jets approaching the island
in a "test" for the new administration of Joe Biden.
On Sunday, 12 Chinese fighter jets entered Taiwan's
air defence identification zone, along with
a reconnaissance aircraft and two anti-submarine
aircraft, Taiwan's defence ministry said.
A day earlier, China sent eight bomber planes capable
of carrying nuclear weapons and four fighter jets
to the same area to the southwest of the island,
as well as one reconnaissance aircraft.
On both occasions, Taiwan sent up aircraft, issued
radio warnings to the Chinese aircraft, and deployed
air defence missile systems to monitor their activity.
jets enter Taiwan air space for second day.
Beijing claims self-governing Taiwan as part of its
territory, and has been angered by a show of
increased US support for Taiwan during Donald
Trump's administration.
In recent months, China has carried out frequent,
at times daily, incursions aimed at pressuring
President Tsai Ing-wen's government to accept
Beijing’s demand that it recognise Taiwan as part
of China.
These incursions have usually consisted of just
one or two reconnaissance planes in recent weeks,
rather than the warplanes seen over the weekend.

The UN aviation agency says the total number of airline passengers
plunged 60 percent last year, as the coronavirus pandemic ground
global travel to a halt.
The International Civil Aviation Organization, or ICAO, says the number
of
domestic and international passengers totaled 1.8 billion in 2020.
That's down from 4.5 billion the previous year.
The spread of the coronavirus prompted governments around the world
to restrict international travel and urge citizens to stay home.
These measures slashed domestic and international air traffic by 50 and
74 percent respectively.
ICAO says the decline cost airlines a combined 370 billion dollars in revenue
and is "threatening millions of jobs around the world."
The agency does say it expects the number of passengers to tick up
by the second quarter of this year. But it adds that recovery will be
"subject to the effectiveness of pandemic management and vaccination
roll out."
 For historic 2nd time
For historic 2nd time
Trump impeached Friday, Jan.15, 2011
House Speaker Nancy Pelosi invoked Abraham Lincoln
and the Bible, imploring lawmakers to uphold their oath
to defend the Constitution from all enemies, foreign
"and domestic."
U.S. President Donald Trump was impeached by
the U.S. House for a historic second time Wednesday,
charged with "incitement of insurrection" over the deadly
mob siege of the Capitol in a swift and stunning
collapse of his final days in office.
With the Capitol secured by armed National Guard
troops inside and out, the House voted 232-197
to impeach Trump.
The proceedings moved at lightning speed,
with lawmakers voting just one week after violent
pro-Trump loyalists stormed the U.S.
Capitol, egged on by the president's calls for them
to "fight like hell" against the election results.
Ten Republicans fled Trump, joining Democrats
who said he needed to be held accountable and
warned ominously of a "clear and present danger"
if Congress should leave leave unchecked
before Democrat Joe Biden's inauguration Jan. 20.
Trump is the only U.S. president to be impeached twice.
It was the most bi-partisan presidential impeachment
in modern times, more so than against Bill Clinton in 1998.
The Capitol insurrection stunned and angered lawmakers,
who were sent scrambling for safety as the mob
descended, and it revealed the fragility of the nation's
history of peaceful transfers of power.
The riot also forced a reckoning among some Republicans,
who have stood by Trump throughout his presidency and
largely allowed him to spread false attacks against
the integrity of the 2020 election.
Why was Trump banned?
Mr Trump was locked out of his account for 12 hours on Wednesday after
he called
the people who stormed the US Capitol "patriots".
Hundreds of his supporters entered the complex as the US Congress attempted
to certify Joe Biden's victory in the presidential election. The ensuing
violence led
to the deaths of four civilians and a police officer.
Twitter warned then that it would ban Mr Trump "permanently"
if he breached the platform's rules again.
US President Donald Trump's Twitter account is "permanently suspended...
due to the risk of further incitement of violence", the company says.
It comes amid a Big Tech purge of the online platforms used by Mr Trump and his supporters.
Some lawmakers and celebrities have been calling for years on Twitter to ban Mr Trump.
Former First Lady Michelle Obama tweeted on Thursday that the Silicon Valley
giants
should stop enabling Mr Trump's "monstrous behaviour" and permanently
expel him.
 I'm a King of F
I'm a King of F?????!!!!!!



US House Speaker Nancy Pelosi says Democratic members of Congress will start
procedures to urge Vice President Mike Pence to remove President Donald
Trump
from office. Pelosi unveiled the plan in a letter to other Democratic lawmakers
on
Sunday, Jan.10, days after Trump supporters laid siege to the US Capitol.
Congress was inside certifying Joe Biden's election victory at the time.
Arnold Schwarzenegger got personal in a video message on Sunday in response
to President Trump’s role in the Capitol riots.
The former California governor opens up about his childhood in post-WWII
Austria,
and says his experience growing up in the aftermath of the Nazi regime have
inspired him to speak out against the President’ adminstration.
Jamie Lee Curtis, Conan O’Brien, and more celebrities have applauded Arnold’s
message.
His children, Patrick and Katherine Schwarzenegger, both shared their father’s video
on Twitter. Patrick called the message, ‘so profound.’

Through the tunnels of the Capitol, senators walked quickly with an armed
police escort.
A member of McConnell’s security detail took the arm of the senator, who
walks
with a limp because of a childhood bout with polio, pulling and steadying him
as they hustled away from danger through a tunnel under the Capitol complex.
Before long, the president’s supporters were inside the Senate chamber,
prowling amid the mahogany desks and even sitting on the marble dais
where Pence had been seated not long before.
Across the Capitol, a police officer stepped onto the House rostrum to inform lawmakers
that they might need to duck under their chairs.
“We now have individuals that have breached the Capitol building,” he told
them,
warning House members to be prepared to move quickly out of the chamber.
“They are in the Rotunda.”


A record 7,841 new coronavirus cases were reported in Japan as of 8 p.m. on Jan.8.
The nationwide daily tally has set a record for a fourth day in a row. Officials
also reported 57
deaths, including 10 in Hokkaido, 7 each in Tokyo, Kanagawa and Aichi,
6 in Saitama, and 5 in Hyogo.
71% of major Japanese companies
expect economy to improve in 2021
Mainichi survey, January 5, 2021
A total of 71% of 118 major Japanese companies
surveyed by the Mainichi Shimbun believe
the economy will improve in 2021, while only 5%,
or six firms, expected worse ahead.
The optimism is founded on projections that,
despite increasing coronavirus cases at present,
things may get better than they were
in the economic deep freeze Japan entered
in spring 2020, when businesses were forced
to curtail their activities as people abided
by stay-at-home requests.
However, in a sign of the continued worries
over the coronavirus's impact, most of the firms
surveyed also said they believed it will take time to
return to pre-pandemic economic conditions.

Japan starts new year amid pandemic
Jan.1, 2021
People across Japan have celebrated the New Year amid
reinforced measures to prevent the spread of the virus.
Meiji Shrine, in Tokyo, opened its gates at 6 a.m.
Masked worshippers were seen keeping a meter apart
from one another to toss coins into a giant offering box.
A woman said, "The staff pay attention to lines
and social distancing. We also tried not to get too close
to each other."
A man said, "I prayed that the coronavirus will subside
quickly so we can once again have time to meet
with other people."
Visitors can buy arrow amulets and good-luck charms
at the shrine, but they're not allowed to touch items
before purchasing them.
Department stores in Japan are gearing up to start
selling special New Year bargains known as "lucky bags".
To avoid congestion, sets containing food items are
sold on a different level from the food section.
The department store put about 70 percent of its lucky
bags on sale at the end of last year as an additional
way of reducing crowds.

Jan.1, 2021, OGA, Akita
While not in full force, the masked ogres came out on New Year’s Eve
to dispel evil and bring good auspices after spritzing their hands with
germ-killing
sanitizer and donning surgical masks beneath their disguises.

Tokyo Metropolitan Government sources say over 1,300 new cases of coronavirus
were
confirmed in the Japanese capital on Dec.31,2020/
That marks the first time the daily tally has risen above 1,000.
The previous high was 949, hit on Saturday.
Infections have been rising rapidly in Tokyo, with recent daily rates
often exceeding 800.
Tokyo Governor Koike Yuriko warned on Wednesday that citizens face an unprecedented
third wave of infections. She said cases could surge at any time, and urged people
to avoid holding parties at home with friends or getting together with groups of relatives.
Koike said infections must be contained over the year-end holidays, and
saving lives
must be the priority. She warned of a possible state of emergency if the
situation worsens.
Temples, shrines have soothing role
to play as pandemic inflames anxiety
December 30, 2020
The impact of the novel coronavirus has spread
to traditional religious events such as New Year's
visits to temples and shrines, funerals and memorial
services for the dead.
Many temples and shrines are in a difficult situation,
but it is hoped that they will continue their activities
while being considerate of people's anxiety.
Ahead of the time for people to make New Year visits,
the Association of Shinto Shrines has drawn up
guidelines calling for such measures as avoiding
crowds and disinfecting objects.
Some temples and shrines call for spreading out dates of
worship rather than concentrating on the first three days
of the New Year.
The number of people infected with the virus
continues to increase, and many railway operators
in large metropolitan areas have decided not to operate
overnight on New Year's Eve through the morning of
New Year's Day.
New Year visits to temple and shrines have served
as opportunities to attract people who do not usually
visit temples and shrines and enable them to feel
a connection with Shintoism and Buddhism.
Therefore, the current situation can be said to
symbolize the hardship of temples and shrines.
Funeral services have also changed due to concerns
about infections. Buddhist rites have been the mainstream,
but it has become necessary to narrow down
the number of mourners present and to refrain
from holding wakes and banquets.
Also, the number of cancellations is reportedly increasing
for such Buddhist occasions as Bon and Higan seasonal
memorial services.
Funerals and memorial services have traditionally
helped in accepting death and healing sorrow.
In recent years, there has been a growing tendency
toward simplification. If the number of temple
parishioners leaving a temple increases, the damage
to the management of the temple, which is
supported by donations, will worsen.
In regional areas, there are many temples and
shrines that continue to function through activities
such as children’s classes to teach good manners
and other topics, and by organizing festivals
with the whole community. The stagnation of such
activities could weaken the bonds that hold local
communities together.
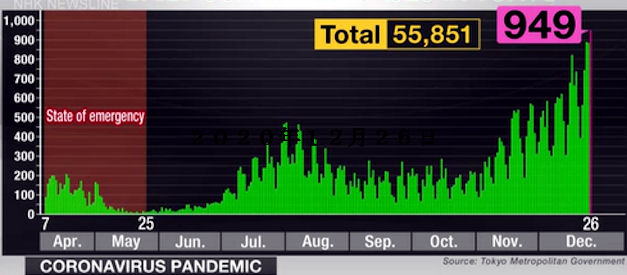
Tokyo reports record 949 coronavirus cases
Saturday, Dec.26, 2020
The Tokyo Metropolitan Government has reported a record 949 new cases of
coronavirus infection in the Japanese capital on Saturday.
The total number of people who have tested positive in Tokyo now stands at 55,851.

Abe escapes indictment
over sakura dinner scandal
Friday, Dec.25, 2020
Prosecutors decided Thursday not to indict former Prime
Minister Abe due to insufficient evidence over alleged political
funding violations related to dinners held on the eve of
cherry-blossom viewing parties.
However, a summary indictment was issued against his
state-paid first secretary, who heads a political support
group for Abe, over irregularities on funds reports.
The Tokyo District Public Prosecutors Office's special
investigation squad filed the summary indictment
regarding Hiroyuki Haikawa, 61, at the Tokyo Summary
Court, over a suspected violation of the Political Funds
Control Law related to the dinners hosted by Abe.
Two others, including the treasurer of a support group
for Abe based in Shimonoseki, were not charged due to
insufficient evidence.
Japan announces ban
on arrivals from Britain
Thursday, Dec.24, 2020
The government on Wednesday announced a temporary
ban on all arrivals of foreigners from Britain, where
a variant of the novel coronavirus is spreading.
The government will also suspend a measure allowing
short-term business travelers returning to Japan from
Britain to be exempt from 14-day.self-quarantine.
Both measures will be effective for the time being
starting Thursday, Chief Cabinet Secretary Kato
told a press conference.
The government hopes to stop the variant, found also
in some other countries, from entering Japan by
strengthening its entry restrictions.
Kato again asked the public to refrain from making
short-term trips to Britain, while indicating
the government's policy of enhancing its quarantine
system.
"We'll take border control measures flexibly,
giving the highest priority toensuring that people
have peaceful and safe year-end and New Year's
holidays," the top government spokesman said.
About whether to tighten restrictions on entry
from other countries, Kato said the government will
"take action swiftly if necessary."
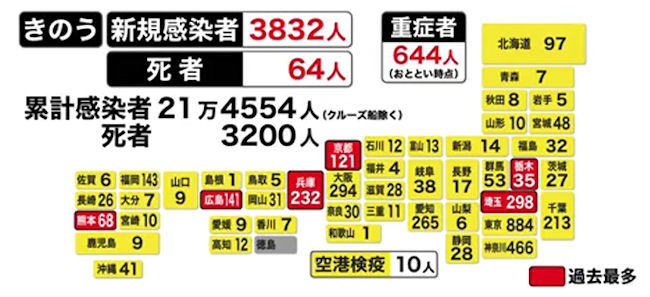
Japan reports
over 2,688 new infections on Tuesday
Wednesday, Dec.23, 2020
Japanese authorities reported 2,688 new coronavirus cases
on Tuesday.They reported 48 deaths, including 12 in Osaka.
The overall total of confirmed cases in the country now
stands at 204,430, including 712 from a cruise ship.
The tally of confirmed deaths has reached 3,026,
including 13 from the vessel.It took only about a month
for the number of deaths to surpass 3,000,
after topping 2,000 in late November.
Health ministry officials say 620 patients were
in serious condition as of Tuesday.A total of
170,001 people, including 659 passengers and crew
members from the cruise ship, have recovered and
left hospitals.
Japanese authorities have reported 2,687 new cases of
the coronavirus as of 8:20 p.m. on Tuesday.
They reported 41 deaths, including 12 in Osaka.
The overall total of confirmed cases in the country now
stands at 204,429, including 712 from a cruise ship.
The tally of confirmed deaths has reached 3,019,
including 13 from the vessel.
It took only about a month for the number of deaths
to surpass 3,000 after topping 2,000 in late November.
Health ministry officials say 620 patients were
in serious condition as of Tuesday.
A total of 170,001 people, including 659 passengers
and crew members from the cruise ship, have
recovered or improved and left hospitals.
Poverty consultations
more than triple in April-Sept.
Dec.21, 2020
Consultation centers in about 900 municipalities
nationwide, where people dealing with poverty
can go for assistance, saw a surge in new inquiries
in the first half of this fiscal year as the novel
coronavirus spread.
In April-September, there were 391,717 new
consultations, more than triple the 124,439
from the same period last year, according to the Health,
Labor and Welfare Ministry.
Because even more people may slip into poverty
at the end of the year, such as through unrenewed
employment contracts, the ministry has asked local
governments to prepare support systems for
the New Year's period.
Last fiscal year, the number of new consultations
was around 20,000 each month.
This fiscal year, the figure jumped to 95,214 and
85,635 in April and May, respectively, during
the nationwide state of emercrency.
The number declined to the 40,000 range in July,
but was higher than 50,000 in August and September.
Now it's on the rise again.
"More and more people are losing jobs or having
their incomes cut due to the effects of the pandemic,"
a ministry official said.
By occupation, many of the people seeking consultations
in the first half of this fiscal year were freelancers,
food service employees and people who work
in the tourism and hospitality sectors, such as hotel
workers.
According to the Japan National Council of Social
Welfare, 185 of the 290 regional councils that operate
aid organizations said at least 10% of the consultations
were from foreigners.
This indicates that many foreigners employed
as dispatch or part-time workers have lost their jobs.
Life of the miserable poor person
An open consultation event was held Saturday
in Tokyo's Hibiya Park to help people who have lost
their jobs due to the pandemic and have fallen
into poverty.
The event was sponsored by an executive committee
made up of civic organizations and labor unions.
To avoid spreading virus infections they did not offer
hot meals, but instead handed out small bags of
food containing rice balls, canned food, fruit and
other items.
"I'll probably be truly homeless in the new year.
At this age, I can't live on the street," said
a 73-year-old man who came to the event.
Saying he had only about \4,000 left, he had
walked from Ueno, where an organization was
providing hot meals, to Hibiya Park.
He had been working part-time cleaning
at a university in Tokyo, but when classes were
suspended due to the coronavirus, his shifts were
cut from five days a week to about one, reducing
his monthly income from about \120,000 to \30,000-
\40,000.
Unable to afford the \60,000 rent for his apartment,
he moved out around July. Since then, he has been
living in a six-tatami mat room at an acquaintance's
place.
"It wasn't even this bad after the Lehman shock,"
he said before making his way out of the park.
A 45-year-old man from Saitama Prefecture
who came to the event said that a dispatch job
he had been promised in March disappeared due to
the pandemic. He has been applying for one or two
jobs a week ever since, but has received no offers.
"I want to work and make money, but because of
the coronavirus, there aren't many jobs being offered.
I don't know what to do," he lamented.
About 50 people came to the event, which included
people in their 30s to 80s, according to the organizer.
"We're worried that over the New Year,
more non-regular workers will not have
their contracts renewed. We want to continue
to support people in need so that they can feel safe
as they welcome the New Year," a person in charge
of the group said.
How did this unbelievable
mix-up of drug ingredients happen?
Dec.20, 2020
In an unbelievable incident, a pharmaceutical company
mixed up the components of a drug with another's,
causing a number of people who took it to lose
consciousness.
It is necessary to thoroughly investigate the cause of
this incident.
An ingredient used in a sleep-inducing drug was mixed
into a medicine manufactured by Kobayashi Kako Co.
in Fukui Prefecture to treat such conditions as
onychomycosis, a fungal disease of the nails.
More than 150 people who took the medicine have
complained of losing consciousness and other
health problems.
Two people have died, and the company is investigating
the causal relationship.
There have also been more than 20 cases of traffic
accidents caused by people driving their cars after
taking the drug.
Kobayashi Kako is voluntarily recalling the medicine,
but the problem is extremely serious.
It is unacceptable that a drug that is meant to cure
a disease has threatened lives.
In an attempt to replenish the medicine's active ingredient,
which was running low during the man-ufacturing
process, an employee reportedly mixed in an ingredient
from a sleep-inducing drug by mistake.
The containers of the two ingredients were different
in size andshape, and it is not usual for the two ingredients
to be mixed up, so the company could only explain it as
grave negligence.
Sloppiness in the procedures was also discovered.
According to the company's inhouse regulations,
a team of two people is supposed to be present
during manufacturing, but there was only one person on site


Govt should take all steps to ensure
prompt vaccinations for coronavirus
Friday, Dec.18, 2020
Vaccinations against the novel coronavirus have begun
in Europe and North America. It is necessary to make
preparations as soon as possible to ensure smooth
vaccinations in Japan as well.
Britain and the United States have started immunizations
using a vaccine developed by Pfizer Inc., a major
U.S. drugmaker.
While no effective cure for COVID-19 has been found,
it can be said that a glimmer of hope is seen at last.
With the arrival of winter, Europe and North America
are facing drastic increases in the number of coronavirus
deaths. Countries are scrambling to give vaccinations
to prevent the further spread of infections through all-out
efforts, even if there are some risks involved.
Doses of the new vaccine are expected to be supplied
to Japan early next year or later. Expectations are
high, but caution is also called for.
It usually takes several years to develop a new vaccine,
but this time, more than one product has been
manufactured at an exceptional pace.
There are many unknown aspects, such as what side
effects they could cause and how long the immunity
conferred by the vaccines will last.
Vaccines developed by Pfizer and U.S. biotech firm
Moderna Inc. use artificially processed ribonucleic
acid (RNA) for the first time. They have been
confirmed to be highly effective in clinical tests,
and no serious side effects have been reported so far.
However, once large-scale vaccinations kick off,
unexpected side effects could be found.
The government should be able to collect promptly
information on side effects at home and abroad
even after vaccinations are started in Japan.
The government is also urged to explain the effects of
vaccines in an easy-to-understand manner.
Tokyo confirms 822 new cases
Friday, Dec.18, 2020
The Tokyo metropolitan government raised the alert
for the city's medical system to the highest of
four possible levels on Thursday due to the spread of
the novel coronavirus, marking the first time it has
done so since the current evaluation system was
introduced in July.
The decision was made at a monitoring meeting with
experts held on Thursday afternoon. It came as
the city set a daily record for newly confirmed
cases, with Tokyo logging 822 cases Thursday,
a significant increase over the previous record of
678 set the day before.
In keeping with the higher alert level, the metropolitan
government will increase the number of hospital beds
reserved for coronavirus patients to 4,000 from
the current 3,000, including an increase of 50 beds
for severely ill patients.
The number of hospitalized patients exceeded 2,000
for the first time on Monday.
On Tuesday, the number of seriously ill people jumped
to 78, the highest level since the state of emergency
was lifted in May.
The alert level regarding the status of infections
in Tokyo has remained at the highest level
on a four-rank scale since Nov. 19.


京都の紅葉80選 : The 80 Best Autumn Leaves Spots In Kyoto.
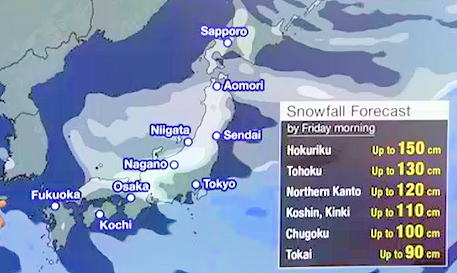
More snow forecast
for Sea of Japan coast
Dec.16, 2020
The season's most powerful cold air mass over Japan is
bringing heavy snow to areas on the Sea of Japan coast
from Hokkaido in the north and to the Chugoku region
in the southwest.
Weather officials are calling for caution against
avalanches, icy roads, and traffic disruptions.
Snowfall in the 24 hours to Wednesday morning
was 128 centimeters in Minakami Town in Gunma
Prefecture, and 113 centimeters in Yuzawa Town
in Niigata Prefecture -- both the heaviest 24-hour
snowfalls on record.
Officials forecast that snowfall in the 24 hours
until Thursday morning will be up to 100 centimeters
in Niigata Prefecture, and 80 centimeters
in the northern Kanto region.
They also expect 70 centimeters of snow on the Sea
of Japan coast of Tohoku, as well as in the Hokuriku
region, Nagano Prefecture and the northern Kansai region.
Officials are warning of traffic disruptions as well as
avalanches and snow accumulation leading
to downed power lines and trees.
Electoral College vote
confirms Biden's victory
Tuesday, Dec.15, 2020
Biden has declared a "clear victory" in Monday's
Electoral College vote.
Biden won support from 306 out of 538 electors,
surpassing the 270 majority needed to formalize
his win.
President Trump secured the 232 remaining votes.
After the results were confirmed, Biden made
a speech in the state of Delaware, declaring victory
and urging Trump to accept the result.
Biden said, "It's time to turn the page as we've done
throughout our history, to unite, to heal." He added
that he will become a president for all Americans and
work just as hard for those who did not vote for him
as he will for those who did.
He also said that there was "urgent work"
ahead for all Americans, in terms of controlling
the pandemic and vaccinating the nation against the virus.
Monday's Electoral College vote is normally a formality.
But it took on added importance this time after
President Donald Trump refused to concede defeat
following the November 3 presidential election.
Biden is expected to be formally elected the 46th US
President by a joint session of Congress on January 6.
Trump has challenged the election results in court.
But with Biden winning a majority of Electoral
College votes, experts say Trump's chances of
overturning the results have become virtually impossible.
Medical fee hike for elderly needed
for sustainable social security system
Monday, Dec.14, 2020
People aged 75 or older with a certain level of income
will have to bear a heavier burden in fees paid at hospitals.
The government must build a sustainable social security
system while carefully seeking their understanding.
The government has decided to raise the percentage of
out-of-pocket payments paid at medical institutions
by elderly people who are 75 and older from 10% to 20%,
for single-person households with an annual income of
\2 million or more.
The government aims to pass a related bill in next year's
ordinary Diet session.
Elderly people in this age bracket are currently charged
10% of the total cost of medical treatment, in principle,
and only those with incomes equivalent to those of
working age are charged 30%.
To create a system in which the burden is not based
on age but on the ability to pay, it is of great significance
that people in the age bracket with moderate incomes
will pay 20%.
In 2022, the large baby-boom generation will begin
to turn 75. The structure of benefits being provided
mainly to the elderly and the burden being borne
mainly by the working-age population needs
to change to one in which all generations support
each other.

Germany to tighten
anti-coronavirus measures
Sunday, Dec.13, 2020
German Chancellor Angela Merkel says
anti-coronavirus measures will be tightened
to stem a surge in new cases across the country.
Merkel told a news conference on Sunday
that most retailers, except for essential stores,
will be closed from Wednesday to January 10.
Schools will also be shut.
A ban on outdoor alcohol drinking will be in effect,
meaning people cannot relish the open-air drinking of
mulled wine, a traditional Christmas beverage
usually served hot.
Merkel said the number of social contacts has risen
during the Christmas shopping season.
She underscored the need for the tougher restrictions
to lessen the burden on healthcare systems.
Bars, restaurants, and other establishments across
Germany have been closed since November 2.
But the coronavirus has continued to spread.
The country confirmed over 29,000 new infections
and 598 deaths on Friday, both record highs.
U.S. & EU economies
have been gripped
by virus pandemic
Saturday, Dec.12, 2020
The worsening of the virus pandemic across
the United States and Europe is threatening
their economies and intensifying pressure
on governments and central banks on both
continents to intervene aggressively.
In a worrisome sign of the harm the virus is
inflicting in the United States, the government
said Thursday that the number of Americans
seeking unemployment benefits jumped last week
to 853,000 -- the most since September.
The surge in jobless claims made clear that
many companies are still shedding workers
as states reimpose business shutdowns and
consumers avoid shopping, traveling or dining out.
Consumers thus far haven't spent as much
this holiday shopping season as they have
in previous years,according to credit and
debit card data, aid last month U.S. employers
added jobs at the slowest pace since April.
Restaurants, bars and retailers all cut jobs
in November.
Responding to similar pressures, the European
Central Bank announced Thursday that it will
ramp up its bond-buying program to try to hold down
longer-term interest rates to spur borrowing and
spending.
The ECB's action coincided with the highest
single-day virus death toll in Germany, Europe's
largest economy, and the shutdown of
restaurants, bars, gyms, movie theaters and
museums in France.
The coronavirus "is having an impact on consumers,
it is having a big impact on the labor force, it is
having an impact on businesses," said Gus Faucher,
an economist at PNC Financial.
"There are reasons to be concerned."
When the U.S. Federals Reserve meets
next week, it may provide more de tailed
guidance on how long it will continue
its own bond-buying program, which could
reassure markets that its purchases won't end
anytime soon.
Over-75s set to pay more
for medical treatment
Friday, Dec.11, 2020
Prime Minister Yoshihide Suga, also president of
the ruling Liberal Democratic Party, and Natsuo
Yamaguchi, head of Komeito, the LDP's coalition
partner, agreed Wednesday to double out-of-pocket
medical expenses to 20% for people aged 75 or
above with annual incomes of Y2 million or more.
Policy chiefs of the two parties will discuss details,
with the aim of making a Cabinet decision
on the matter on Tuesday.
The government plans to raise out-of-pocket medical
costs to 20% from 10% for people in the age group of
75 or over in fiscal 2022.
Specifically, the increase is expected to be implemented
in or after October 2022, after a triennial election
for the House of Councillors in summer that year,
as sought by Komeito, sources said.
LDP-Komeito talks on the issue had been deadlocked.
Suga had insisted that the annual income threshold
be set at \1.7 million.
On the other hand, Komeito had called for the line
to be drawn at \2.4 million, claiming that Suga's
proposal would raise the burden for too many people.
Suga and Yamaguchi saw the need to end the row
early because the two parties are also at odds
in coordination work over whom to field in the No. 3
constituency in Hiroshima Prefecture in the next
general election for the House of Representatives
after Katsuyuki Kawai, who is now the Lower
House member elected from the single-seat
electoral district, left the LDP in June over his
alleged violation of the public offices election law.
Kawai is standing trial for suspected vote-buying
for his wife; Anri, for the Upper House election
in July 2019.
Medical costs in the country are highly likely
to balloon in and after fiscal 2022, when baby-
boomers start to turn 75.
Currently, people aged 75 or over who earn
\3.83 million a year pay 30% of their medical
treatment costs by themselves.
But they account for only 7% of all people
in the age group.
The annual income threshold of \million will
make 3.7 million people subject to the envisaged
hike in out-of-pocket expenses, fewer than some
5.2 million people under the Suga-proposed
threshold of \1.7 million, resulting in reduced
effects in easing the related financial burdens
on working generations.
15 key areas picked
to meet carbon goal
Friday, Dec.11, 2020
In an effort to achieve virtually zero greenhouse
gas emissions by the year 2050, the government has
designated 15 priority areas, including increasing
the capacity of offshore wind power generation
to a maximum of 45 million kilowatts by 2040,
equivalent to the capacity of 45 nuclear power plants.
The outline of the government's plan for its "green
growth strategy" aimed at achieving carbon neutrality
by 2050, which was revealed Wednesday, will be
discussed at an inter-ministerial panel of experts.
The aim is to finalize the plan at the government's
Committee on the Growth Strategy by the end of
this year.
The plan specifies the targets and necessary
institutional arrangements for each of the priority
areas in three stages:
research and development,
demonstration, and
wide-range implementation.
The government's current target for the capacity of
offshore wind power generation is 10 million kilowatts
by 2030, but this will be raised to a level of 30 million
to 45 million kilowatts by 2040.
This would make Japan's capacity one of the largest
in the world, behind Europe and China.
There are no manufacturers in Japan that produce
the key components for wind power generation.
The government aims to foster related companies
and increase domestic procurement to 60%,
including operation of the facilities, as well as
expand local employment.
The government aims to commercialize in the 2030s
a floating offshore wind system that can generate
power on the sea, eventually exporting the technology
to make it a key Japanese industry.
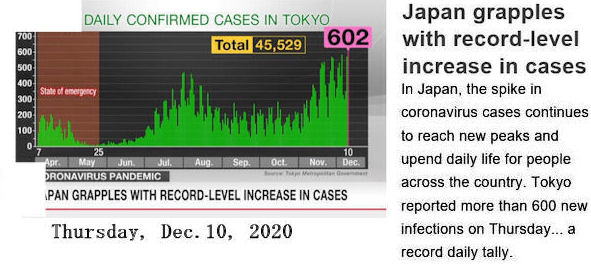
Govt plans huge additional spending
Thursday, Dec.10, 2020
An additional economic package approved by the Cabinet
shows that the government has decided to continue
with massive fiscal stimulus.
While the package aims to shift focus toward structural
changes to society and the economy for after
the pandemic, there are concerns that a course
correction may be needed depending on how
the novel coronavirus spreads.
Ruling bloc takes lead "A fund in the 110 trillion
range is sought as soon as possible," reads
the text of the additional stimulus package released
Tuesday, giving a clear numerical target for a fund
to support university research and development.
The third supplementary budget for fiscal 2020 and
treasury investments and loans for fiscal 2021
amount to about 14.5 trillion, with the goal to
reach 110 trillion in stages.
Last week, a draft economic policy package distributed
by the Liberal Democratic Party's executive committee
only included the abstract phrase "[a fund] on the scale
of othersaround the world."
Led by Akira Amari, chairperson of the LDP's Research
Commission on the Tax System, who conveyed the support
of Prime Minister Yoshihide Suga, the wording was changed
at the last minute before the Cabinet decision.
Discussions on additional economic stimulus and
a third supplementary budget began in mid-October
within the government and ruling parties.
The Finance Ministry envisioned limiting the supplementary
budget to a few trillion yen, as in most years.
However, after a level period, the number of infections
in Japan began rising in late October.
Once November came around, daily records were being
broken regularly.
Around that time, arguments for in-creasing
the third supplementary budget began gaining steam
in the ruling parties, with those calling for "110 trillion
to 115 trillion" becoming dominant.
Noting that the shortfall in domestic demand grew
to 134 trillion in the July-September period, LDP Policy
Research Council Chairman Hakubun Shimomura asked
Suga at the end of November for "a large supplementary
budget that will come close to filling that in."
The final figure ended up at 119.2 trillion.
With about 125.6 trillion from the general account
in the first supplementary budget and 131.9 trillion
in the second, expenditures for fiscal 2020 will
approach 1180 trillion when the third supplementary
budget is included.
This is 1.8 times the original budget, and there is
growing concern about deteriorating fiscal discipline
from issuing more bonds to finance it.
Britain starts mass vaccinations!
Wednesday, Dec.9, 2020
Britain began the mass vaccination of its population
against COVID-19 on Tuesday, becoming the first
Western nation to do so in a global endeavor that
poses one of the biggest logistical challenges in
peacetime history.
On a day dubbed "V-Day," health workers started
inoculating people with a shot developed by Pfizer
and BioNTech, with the country a test case
for the world as it contends with distributing
a compound that must be stored at minus 70 C.
Britain is the first country globally to begin mass
inoculations with that vaccine, one of three shots
that have reported successful results from large
trials after being developed in record time.
Margaret Keenan, a 90-year-old grandmother
from Britain, was the first person in the world
to receive the vaccine outside of a trial following
its rapid clinical approval.
Health Minister Matt Hancock described the start
of vaccinations as "V-Day." Hancock said,
"If we manage to do that for everybody who is
vulnerable to this disease, then we can move on
and we can return to normal."
The launch will fuel hope that the world may be
turning a corner in the fight against a pandemic
that has killed more than 1.5 million people and
crushed economies.
Britain, the worst-hit European country with more
than 61,000 deaths, has ordered enough supplies of
the Pfizer-BioNTech shot to vaccinate 20 million
people.
The developers said it was 95% effective in preventing
illness in final-stage trials.
In Britain, about 800,000 doses are expected
to be available within the first week, with care home
residents and carers, the over-80s and some health
service workers prioritized.
Roosevelt's
the "Day of Infamy" speech
President Roosevelt delivers the "Day of Infamy" speech
to a joint session of Congress on December 8, 1941.
Behind him are Vice President Henry Wallace (left) and
Speaker of the House Sam Rayburn.
To the right, in uniform in front of Rayburn,
is Roosevelt's son James, who escorted his father
to the Capitol.
韓国従軍慰安婦問題
When Roosevelt delivered the speech, most of his
on-the-spot changes involved word order.
But many people had never heard of Oahu,
the Hawaiian island on which Pearl Harbor and
Honolulu are located, so it became "the American
island of Oahu" to establish the fact that
America had been attacked.
And the sentence "Very many American lives
have been lost" became "I regret to tell you
that very many American lives have been lost."
In fact, 2,403 Americans died in the attack.
You Tube: ルーズベルト大統領の戦争宣言演説 7分38秒
The success of the Pearl Harbor
surprise attack was a fixed game.
I am surprised by terribleness of the applause of
members of the Diet whenever I hear this speech.
As it is in this speech,
The surprise attack was carried out islands of
the Pacific and Southeast Asian whole area
as well as Pearl Harbor.
The supreme leader of Japanese army and navy
were not thinking at all so that they were not able
to imagine the anger of the American nation
for the surprise attack to become terrible.
In neither the Japanese Government at the time
nor the army and navy, the Japanese supreme leader
at the time speak an opinion about nobody,
the United States and the nation feelings of China
including Emperor Showa, Isoroku Yamamoto.
The large-sized newspaper which fell in the order
organization of former Imperial Army does not give
an opinion about the United States and the nation
feelings of China.
The whole country of Japan was lack of sense
of 100% information about the nation feelings of
the other country.
The supreme leader of Japan ignorant at the time of
1941 and they thought that there were a peace
to too stupid Japanese Government at the time,
the supreme leader of army and navy at a certain
point in time and decided the outbreak of war to U.S.
It was in that even peace with the Chinese
Chiang Kai-shek Administration was not possible.

Hayabusa2 capsule retrieved
Dec. 8, 2020
Japan's space agency JAXA has retrieved a capsule
released from its asteroid probe Hayabusa2.
Hayabusa2 released the capsule believed to contain
sand samples from the remote asteroid Ryugu
on Saturday Japan time.
The 5-billion-kilometer voyage to and from Ryugu
that took six years has come to an end.
Hayabusa2 is now heading for another asteroid on
a new mission. It is expected to arrive there in 11 years.
Wall St. hits highs as
slowing job growth
spurs stimulus bets
Sunday, Dec. 6, 2020
Wall Street's main indexes rose to all-time highs Friday
as data showing the slowest U.S. jobs growth
in six months raised investors' expectations for
a new fiscal relief bill to help revive the coronavirus-hit
economy.
So-called cyclical stocks seen as particularly sensitive
to the economy, such as energy, materials and industrials,
shined as most S&P 500 sectors rose.
The U.S. Labor Department's closely watched report
showed nonfarm payrolls increased by 245,000 jobs
in November, below economists' expectations of
469,000 jobs and the smallest gain since the labor
recovery started in May.
"The bad news of the weakening jobs picture is
potentially good news for investors, because
it means that the stimulus bill is much more likely
to take place in a fairly short time frame," said
Ryan Detrick, senior market strategist at LPL
Financial in North Carolina.
The Dow Jones Industrial Average rose 248.74 points,
or 0.83%, to 30,218.26, the S&P 500 gained 32.40
points, or 0.88%, to 3,699.12 and the Nasdaq
Composite added 87.05 points, or 0.7%, to 12,464.23.
The Dow Jones Transportation Average and
the small-cap Russell 2000 also posted record
closing highs.
The benchmark 10-year yield hit its highest level
since March at over 0.98%, helping support
financial shares which are highly sensitive
to interest rates.
The energy sector jumped 5.4%, bolstered by gains
in oil prices.
Shares of Diamondback Energy Inc surged 12.7%
and Occidental Petroleum gained 13.4%.
"There is just a lot of catch-up happening
with those sectors and subsectors that have really
struggled year to date," said Eric Freedman, chief
investment officer at U.S. Bank Wealth Management.
Osaka court rejects
Oi plant nuclear reactor
Sunday, Dec.6, 2020
The Osaka District Court on Friday nullified the Nuclear
Regulation Authority's judgment that two reactors
at Kansai Electric Power Co.'s Oi nuclear plant
in central Japan meet safety levels required by law
for restarts.
In a lawsuit, 127 residents in 11 prefectures
including Fukui, which hosts the plant, demanded that
the NRA's judgment allowing the plant's No. 3 and
No. 4 reactors to restart be canceled, claiming
that there are problems with earthquake resistance
and other safety measures for the facilities.
Handing down the ruling, presiding Judge Hajime Morikagi
said that "there are unreasonable points" about
the NRA's judgment.
The ruling was the first to cancel the NRA's approval
for alterations to reactors to meet thecountry's
new safety standards, drawn up in response
to the March 2011 triple meltdown at Tokyo Electric
Power Company Holdings Inc.'s tsunami-stricken
Fukushima No. 1 plant in northeastern Japan.
The ruling is expected to have far-reaching implications.
The NRA uses the same method to check the safety
levels of other reactors.
'The NRA evaluates the reasonableness of a "design
basis earthquake ground motion," or the level of
shaking used as a standard in designing earthquake-
resistant structures, set for a nuclear plant
by a power company based on the new nuclear safety
standards intro-duced in 2013.
It approves the restart of a reactor that meets the standards.
The main issue in the suit was whether Kansai Electric
set a reasonable design basis earthquakeground motion
for the Oi reactors.
Morikagi said the new standards call for taking
into account the possibility of bigger earthquakes
than forecast and possible deviations from the average
forecast.
In the NRA's screenings, there were errors and
insufficiencies that cannot be overlooked, the judge
said, noting the failure to take such deviations
into consideration.
The NRA's judgment "is illegal because it did not check
what it must," Morikagi said.
The government said that the reactors' earthquake
resistance was far above a sufficient level and that
their safety functions would not stop working immediately
even if a temblor larger than the design basis earthquake
ground motion occurred.
In 2013, Kansai Electric applied for NRA screenings of
the two Oi reactors dnder the new
Global virus toll passes 1.5 million
Sunday, Dec. 6, 2020
The world passed the grim milestone of 1.5 million
coronavirus deaths on Thursday, as several nations
planned to deliver much-hoped-for vaccines early
next year to break the cycle of lockdowns and restrictions.
U.S. President-elect Joe Biden said that on his first day
in office he would ask Americans to wear masks for
100 days to help reduce transmission of the virus
that is again surging in the country with the world's
highest number of deaths and infections.
"I'm going to ask the public for 100 days to mask.
Just 100 days to mask -- not forever," Biden said
in excerpts of an interview for CNN.
But even as the latest positive news about a vaccine
was announced, with the Moderna candidate showing
it confers immunity for at least three months, several
countries marked new COVID-19 records.
The United States, for instance, posted an all-time high
of more than 210,000 new cases in a 24-hour stretch
to Thursday evening, meanwhile notching more than
2,900 deaths, ac-cording to Johns Hopkins University.
And Italy registered 993 deaths, topping its previous
record of 969 earlier in the year when it was the first
European country to be affected by the pandemic.
To build trust in vaccines after they are approved,
the 78-year-old Biden said he was willing to be
vaccinated in public -- following up on similar
commitments from former U.S. Presidents
Barack Obama, George W. Bush and Bill Clinton.
Biden also used the interview to say he had asked
the government's top infectious disease specialist
Anthony Fauci to join his COVID team and serve
as a chief medical adviser.
`Maximum caution' urged
Saturday, Dec.5, 2020 from the Japan news
"Maximum caution is necessary," a Health, Labor and
Welfare Ministry advisory body on the novel coronavirus
said Thursday, pointing to various "record-high levels."
It also said the amount of inpatients and seriously
ill patients has been on the rise, making it hard
to manage hospital space in some areas.
According to the ministry's statistics, the number of
newly infected people per 100,000 nationwide was
8.95 in the week ending Nov. 18, but it increased
to 12.37 in the week ending Wednesday.
The average effective reproduction rate, which indicates
the average number of people that one individual infects,
exceeded 1.0 -- considered the threshold for an accelerated
spread of the virus -- for the week ending Nov. 17
in Hokkaido, Tokyo, Aichi and Fukuoka and other
prefectures.
As of Tuesday, the occupancy rate of hospital beds
for COVID-19 patients exceeded 50% in Hokkaido,
Osaka and Hyogo prefectures.
The advisory body said that in many cases, people
in their 20s to 50s who have traveled across prefectural
borders have infected others with the virus.
Takaji Wakita, director of the National Institute of
Infectious Diseases who chairs the advisory body,
said, "Young people and those in their prime
must be urged to wear masks when traveling or eating."
In an effort to avoid a medical crisis, the ministry
encouraged prefectural governments to quantify
the risks of becoming seriously ill for patients based
on their symptoms, age, record of chronic diseases
and other factors to determine whether they should
be hospitalized.
It aims to narrow down the intake of patients
to effectively manage hospital capacity.
Japan must create society
in which older people
can actively contribute
Dec.4, 2020 from the Japan news
Respect for the Aged Day falls on the third Monday
in September. It is hoped that a society will be created
in which people can undertake various challenges
in keeping with their wishes and physical strength,
regardless of age.
According to the Internal Affairs and Communications
Ministry and other sources, the number of people
aged 65 or older has hit a record high of 36.17 million
in Japan.
They account for 29% of the nation's population.
The number of people aged 100 or older has exceeded
80,000 for the first time.
It is wonderful that Japan has some of the greatest
longevity in the world.
Even aged over 100, some people give lessons
on how to make hand-made udon noodles, while
others teach the traditional Japanese tea ceremony or
watch over children.
If more elderly people with a wealth of experience
and knowledge take part in society, they will help
revitalize local communities. There are many things
that younger generations can learn and much
encouragement they can receive from elderly people.
To further boost the number of areas where people
can play an active role, it will likely be necessary to
review the attitude that classifies people aged 65 or
over as elderly and regards them as supported by
society.
This age classification in population statistics is said
to be attributed to a report released by the United
Nations in the mid-1950s, in which people aged 65 or
older were categorized as elderly.
The average life expectancy of Japanese men has
jumped from 64 to 81, and that of Japanese women
from 68 to 88.
It was reasonable for the government to point out
in its 2018 Guideline of Measures for Aging Society
that the general trend of classifying people over 65
as "older people" is no longer realistic.
The government also set a goal of extending
people's healthy life span, the years in which
people can livetheir daily lives without problems.
Global coronavirus cases
surpass 60 million
Friday, Nov.27, 2020 from the Japan news
The global tally of confirmed coronavirus cases
hit 60 million on Wednesday, with the pace of
new infections accelerating and the United States
reporting record numbers of hospitalizations,
according to a Reuters tally.
Officials in the United States, the worst-affected
country in the world, urged Americans to stay home
for the Thanksgiving holiday as soaring numbers of
COVID-19 patients pushed medical professionals
to the brink.
The United States has reported 1 million new COVID-19
cases in less than a week, taking its total reported
infections to over 12.5 million and its death toll
to 260,000, according to the Reuters data based
on official statements.
Globally, infections stood at 60.005million and
deaths at 1.4 million.
An analysis of the Reuters data showed the rate of
new infections picking up globally.
It took just 17 days to go from 50 million cases
to 60 million, compared with the 21 days it took
to go from 40 million to 50 million.
Around 580,000 cases have been reported each day
over the past week.
Can an explosion of infections
be contained or not?
Nov.26, 2020 from the Japan news
This is a critical moment. The government, businesses
and the public must share a sense of urgency and
do everything in their power to address the crisis.
The number of novel coronavirus infections has
exceeded 2,000 new cases per day across the country,
a record high.
The number of new daily cases in Tokyo exceeded 500,
prompting the metropolitan government to raise its
coronavirus alert to the highest level, indicating
"the infection is spreading."
The coronavirus pandemic could be viewed as having
entered a different phase from before.
This sudden expansion of infections is different
in nature from that of the "second wave" in summer.
In Tokyo, infection clusters have not been confined
to nightlight districts, but have also spread to workplaces
and university dormitories. Household infections also
account for more than 40% of the total.
The number of people whose route of infection is
unknown has reportedly increased.
There appear to be many cases of people, including
asymptomatic cases, who have been unwittingly
spreading the virus.
It is necessary to continue strengthening follow-up
investigations by public health centers and conducting
intensive PCR (polymerase chain reaction) tests
in areas where infected cases are found.
It is worrisome that cases have been conspicuous
among elderly people, who are more prone to become
seriously ill.
The bed utilization rate in the whole country has
been rising. If infection numbers continue to rise,
it could lead to a scarcity of hospital beds and cripple
medical systems in regional areas.
The central and prefectural governments need
to steadily expedite measures not only to secure
hotels and other accommodation facilities
for people with mild symptoms, but also to strengthen
the function of medical institutions.
Revisions to Go To campaigns
Nov.25, 2020 from the Japan news
It is only natural for the government to change its policy
flexibly depending on the status of novel coronavirus
infections.
Every possible measure should be taken to prevent
confusion from spreading.
The government has announced revised measures
for the operations of its Go To campaigns to stimulate
demand.
The government has decided to suspend subsidies
for both new and existing reservations for trips
to destinations with infection spikes under
the Go To Travel domestic tourism promotion campaign.
The cities of Sapporo and Osaka will be excluded
from the campaign until Dec. 15.
Hospital bed utilization rates and the percentage of
patients whose routes of infection are unknown
have been rising in Tokyo and other urban areas,
leading some experts to believe that the infection
alert status is approaching Stage 3, the second most
severe level.
While it is necessary to balance economic activities
and infection prevention measures, now is the time
to shift the emphasis to curbing the spread of infections.
It is important for the government to send a strong
message to the people and call for their cooperation.
Companies must strive
to reform and develop businesses
amid pandemic
Nov.24, 2020 from the Japan news
There are signs of a turnaround in corporate earnings,
which have been adversely impacted by the novel
coronavirus pandemic.
However, there is also a clear polarization. Companies
that are suffering must overcome their difficulties
by reforming and creating new businesses.
The announcement of half-year business results
to September by companies listed on the Tokyo Stock
Exchange has passed its peak.
The combined net profits of the 1,217 companies listed
on the First Section of the Tokyo Stock Exchange,
excluding the financial sector, fell about 40%
from a year earlier.
The situation has picked up since the April-June quarter,
in which net profits fell by about 60% due to
the declaration of a state of emergency and other factors.
For the full year ending in March 2021, only a 20%
year-on-year decline is said to be expected.
Noteworthy is the rebound in the manufacturing industry
due to the recovery of overseas markets such as China.
In the auto industry, a number of companies raised
their earnings forecasts for the business year ending
in March 2021. The turnaround of a core industry
that has a trickle-down effect to other industries is
positive news.
Toyota Motor Corp. raised its net profit estimate from
\730 billion to \1.42 trillion. In addition to a recovery
in overseas sales, steady efforts to improve efficiency
will also have an impact.
Honda Motor Co. revised up its forecast for the business
year ending in March 2021 to \390 billion.
It is expected that the situation will have a ripple effect
on their business partners such as parts suppliers.
Changes in lifestyles caused by the novel coronavirus
pandemic have been turned into opportunities
by many companies.
Preventing spread of coronavirus
Nov.23, 2020
Debate in the Diet between the ruling and opposition
parties over issues related to the novel coronavirus
pandemic did not go well.
The government should present clear guidelines
to the public about how to overcome this crisis.
The budget committees of the House of Representatives
and the House of Councillors held intensive deliberations.
When it came to measures against infection,
the opposition parties focused on the government's
Go To Travel tourism promotion campaign.
Yukio Edano, leader of the Constitutional Democratic
Party of Japan, called for the campaign to be suspended,
saying, "Infections spread when more people travel."
Prime Minister Yoshihide Suga countered, "There is
no evidence that [the Go To Travel campaign] is
a major cause of the spread of infections. It is
a powerful measure that can bolster the economy."
It is highly important for the government's support
measures to underpin the tourism industry, which
has been hit hard by the pandemic.
There must be many companies that can keep
operating thanks to the positive effects of the campaign.
However, is it appropriate for the government
to continue encouraging the public to travel even
when the number of cases is surging?
While calling for restraint regarding trips to destinations
where infections are spiking, the government still
continues to provide subsidies for residents of those
areas to travel to other places. It is hard to ignore
the inconsistency in this approach.
Asked why the number of cases is surging, Health,
Labor and Welfare Minister Norihisa Tamura said,
"More people are traveling without sufficient measures
against infection in place."
In a bid to prevent society from lowering its guard,
the government should once again urge the public
to thoroughly follow basic protocols, such as wearing
masks and avoiding the so-called Three Cs of closed
spaces, crowded places and close-contact settings.
The number of seriously ill patients in Japan has been
hitting record levels, with the occupancy rate of
hospital beds also rising in urban areas.
There is an urgent need to expand PCR (polymerase
chain reaction) tests and medical services.
Corporate earnings
Nov. 18, 2020
There are signs of a turnaround in corporate earnings,
which have been adversely impacted by the novel
coronavirus pandemic.
However, there is also a clear polarization. Companies
that are suffering must overcome their difficulties
by reforming and creating new businesses.
The announcement of half-year business results
to September by companies listed on the Tokyo Stock
Exchange has passed its peak.
The combined net profits of the 1,217 companies listed
on the First Section of the Tokyo Stock Exchange,
excluding the financial sector, fell about 40% from a year
earlier.
The situation has picked up since the April-June quarter,
in which net profits fell by about 60% due to the declaration
of a state of emergency and other factors.
For the full year ending in March 2021, only a 20%
year-on-year decline is said to be expected.
Noteworthy is the rebound in the manufacturing industry
due to the recovery of overseas markets such as China.
In the auto industry, a number of companies raised their
earnings forecasts for the business year ending
in March 2021. The turnaround of a core industry
that has a trickle-down effect to other industries is
positive news.
Toyota Motor Corp. raised its net profit estimate
from Y730 billion to Y1.42 trillion. In addition to
a recovery in overseas sales, steady efforts to improve
efficiency will also have an impact.
Honda Motor Co. revised up its forecast for the business
year ending in March 2021 to Y390 billion.
It is expected that the situation will have a ripple effect
on their business partners such as parts suppliers.
Changes in lifestyles caused by the novel coronavirus
pandemic have been turned into opportunities
by many companies.
Japan's nuclear power
Nov.17, 2020
Tohoku Electric Power Co.'s Onagawa nuclear power
plant has taken a giant step toward resuming its operations.
The government should make progress in restarting
other nuclear power plants step by step while confirming
their safety.
Miyagi Gov. Yoshihiro Murai has expressed his intention
to approve the restart of the No. 2 reactor at the Onagawa
plant after holding talks with the mayors of the two
municipalities in the prefecture that host the nuclear power
plant - the town of Onagawa and the city of Ishinomaki.
It is commendable that the governor carefully compiled
opinions based on the desires of the prefectural, town
and city assemblies.
Tohoku Electric aims to restart the reactor in fiscal 2022
or later, when safety measures are set to be completed.
Among the reactors affected by the 2011 Great East
Japan Earthquake and tsunami, the No. 2 reactor
at the Onagawa plant became the first to win restart
approval from local municipalities.
It is also significant from the viewpoint of a stable power
supply.
The Onagawa plant has implemented various safety
measures since the disaster. Tohoku Electric is building
a seawall that reaches 29 meters above sea level,
installing power-supply vehicles and conducting
earthquake-resistance work at the plant.
These efforts were recognized and the reactor passed
the Nuclear Regulation Authority's safety screening.
However, there are many narrow, winding roads along
the jagged coastline near the plant. Some residents are
said to be worried about whether they will be able
to evacuate smoothly in the event of an accident.
It is important for the central government to continue
improving evacuation measures in cooperation
with local governments.
China going forward
to make Hong Kong change
Nov.16, 2020
China has set new conditions for membership of
Hong Kong's Legislative Council. Conditions stipulate
that members will be immediately disqualified
for supporting "Hong Kong independence," seeking
sup-port from foreign powers or conduct deemed to lack
"loyalty" to the Hong Kong government.
Based on the new rule, the Hong Kong government
dismissed four pro-democracy lawmakers from the council.
Under Hong Kong law, the disqualification of a council
lawmaker requires the approval of two-thirds of council
members or a court decision, but the government has
now grabbed the power to do so.
The move follows the introduction in June of China's
national security law to crack down on anti-government
activity in Hong Kong, a measure that further tightens
the grip on the pro-democracy bloc.
Unilaterally disqualifying elected lawmakers is a denial of
the principles of the election system andseparation
of three powers.
The criteria for determining the presence or absence of
"loyalty" are extremely vague and are likely to be used
arbitrarily.
It is possible that lawmakers viewed as lacking loyalty
will lose their jobs for simply expressing objections
to the government on the council floor.
Under such circumstances, the council will lose its ability
to monitor administrative errors and its independence,
and will become a supporting organization that only
approves government policies.
"We can no longer tell the world that we still have
'one country, two systems.' This declares its official
death," a senior official representing pro-democracy
lawmakers said.
In addition to the four lawmakers who were disqualified,
15 prodemocracy lawmakers tendered their resignations
in protest. This left the council in an unprecedented
situation, with pro-China lawmakers occupying the majority
of the seats.
The number of the illegal
Chinese prostitutes is 20 million.
Record China
It is said that the number of the Chinese prostitutes
comes up in 20 million estimates.
The prostitution is illegal, and the law enforcement
authority supervises it strictly.
However,the "demand" expansion does not stop,
and the number of the prostitutes continues increasing.
The prostitution is illegal, but the prostitution already of
China big; become industrial. It can be said that
the "sex large country" of recent China yellowtail is
unprecedented.
I sell a body than I am poor, and trends to have
brand-name products prevail.
In November, 2019, a collective prostitution by 20
female junior and senior high school students was found.
Until several years before just 30, the problem of the nature
was considered to be a taboo.
Any resistance loses it in "I buy a woman for money"
"selling a body" now.
China seemed to be made to pay compensation called
the large degeneration of the sex morality in exchange
for rapid economic development.
The VIP of the Chinese Communist Party never detects it,
but prostitution is China and is the big fact that is well-known
to becoming industrial.
As for them, "a hair salon" makes "a massage parlor"
the workplace "a karaoke" "a nightclub" "a hotel".
The authorities destroy it frequently and campaign and
disclose a prostitute and prostitution visitors, but still there is
no sign that prostitution, prostitution decreases at all.
Plum Milky Way (Lee in Ho) he of the well-known gender scholar claims,
"you legalize prostitution rather and should detect them as asexual
intercourse worker socially".
The current sex ratio is 120 to 100. A single person will be forced
to about 30 million men of the marriageable age throughout the life.
In other words, the man only for it will have a problem of the nature
in distress. According to the investigation that Mr. plum Milky Way
performed in a farm village of Hunan, the only method that a one-third
solved a problem of the nature as an unmarried person of the man
was prostitution.
To the net top and a newspaper, a magazine, the obscene words such as
a "girl complete nudity" "lechery" "metamorphosis" overflow.
A stimulating word such as "confidence of captain and CA" "immorality" is
founded in the media firm such as state-run Xinhua News Agency and
Chinese Communist Party bulletin, Renmin Ribao. the government official
who brandished power, and kept some lovers was not a rare person anymore



Novel coronavirus pandemic
Nov.15, 2020
Novel coronavirus infections are surging again.
The government should take this matter seriously
and take every possible measure to address the situation.
The number of new daily cases in Japan has reached
a record-high level. Noteworthy is that infections are
spreading across the country, including in Osaka Prefecture
and Hokkaido, in addition to Tokyo.
Experts believe that this is the "third wave" of the pandemic,
following the outbreak early in the year and a resurgence
in summer.
Vigilance must not be neglected amid the approaching
winter season, when people are likely to spend more
time indoors, in poorly ventilated spaces.
The government has asked universities and workplaces
to exercise caution with regard to drinking parties and
dormitory life in order to strengthen measures against
infection clusters.
The government also plans to ask restaurants to ventilate
and control humidity levels.
Information for foreigners will apparently be disseminated
in multiple languages. How to enable technical interns
and others who live in groups to avoid crowded settings is
an important issue that must be thoroughly addressed.
There have been endless cases of asymptomatic young
people transmitting the virus to elderly people and others
who live with them.
People should thoroughly implement basic measures,
such as wearing masks and avoiding the so-called
Three Cs of closed spaces, crowded places and
close-contact settings.
The development of a vaccine has reportedly entered
the final stage in the United States.
However, it will likely take some time before many
people in Japan can receive the vaccination.
Social security system
Nov.14, 2020
How should a system be built to gain the trust of all
generations while limiting the rising burden
on the working population?
This needs to be considered from multiple perspectives.
A government panel on social security for all generations
has been discussing relevant issues ahead of publishing
its final report at the end of the year.
The burden on the working-age population to support
the social security system is expected to become
even heavier after 2022, when members of
the baby boom generation begin to turn 75.
One urgent task is to review the conventional structure,
in which benefits are provided mainly to the elderly and
the burden is mostly borne by the working population.
The focus is on the percentage of out-of-pocket medical
expenses borne by the elderly. For those aged 70 to 74,
this figure had been 10% in principle, but since 2014
the government has gradually raised it to 20%.
Now, those aged 75 and older are subject to the revision.
At present, that rate is still set at 10% in principle,
while those with high incomes are required to pay
30% of the expenses.
The government is discussing specific income criteria,
with a view to raising the rate to 20% for those
with moderate incomes.
It is unavoidable to ask the economically able elderly
to bear their fair share of expenses in order to increase
the sustainability of the system.
Another expected effect would be curbing the excessive
rise in medical costs.
The government should carefully build up a data-based
debate on the impact the increased burden may have
on the elderly's access to health care and their household
finances.
Compensation to former
requisitioned workers
Nov.13, 2020
Two years have passed since South Korea's Supreme
Court handed down rulings ordering Japanese
companies to pay compensation to former
requisitioned workers and others, but the South Korean
government continues to leave the matter untouched.
Prime Minister Yoshihide Suga met with South Korea's
National Intelligence Service Director Park Jie-won during
his visit to Japan, calling on him to take steps to resolve
the issue.
Park is said to have proposed drafting and announcing
a new declaration following up on the 1998 Japan-South
Korea Joint Declaration.
The Moon administration apparently wanted to show that
it was willing to improve relations with Japan, taking
advantage of the inauguration of the Suga administration.
However, public opinion in Japan toward South Korea
has hardened as the South Korean government has
persisted in rehashing the issue of so-called comfort
women and other matters, and fueled anti-Japanese
sentiment.
The 1998 joint declaration was groundbreaking in that
it aimed to put an end to historical issues and emphasized
a future-oriented bilateral relationship.
Under the current circumstances, in which there is
no prospect for a solution to the issue of former
requisitioned workers and the Japanese side is
increasingly distrustful of South Korea, discussing
a new declaration would be putting the cart before
the horse.
Following the Supreme Court rulings, procedures are
underway in South Korea to sell assets held by
the Japanese companies. If the assets are converted
into cash, the Japanese government intends to
implement strong countermeasures, claiming that
conversion would violate the 1965 Agreement
on the Settlement of Problems concerning Property
and Claims and on Economic Cooperation between
the two countries, which stipulates that the matter of
claims has been resolved.
The purpose of digitization
Nov.12, 2020
Amid the coronavirus crisis, Japan's delay in digitization
has been revealed in such ways as through the confusion
that arose over online applications for the provision of
cash benefits of \100,000 per person.
The administration of Prime Minister Yoshihide Suga
regards the promotion of digitization as a key policy issue.
As a precondition for achieving this goal, it is necessary
for the public to widely use personal computers,
smartphones, the internet and other means to realize
the convenience of digital services.
The most important thing is to give consideration
to elderly people who are not familiar with these digital
devices.
According to a survey by the Internal Affairs and
Communications Ministry, the percentage of internet
users among those 65 or older was lower than among
other age groups.
Even if they used the internet, about half of the people
in that age range did not use it frequently and could
not make full use of it.
While some people do not feel the need to use digital
devices in their daily lives, there may be many people
who do not have anyone around whom they can consult
about how to use them.
Some people may not be able to easily ask because
their families live elsewhere.
It is important to take measures not to leave
these people behind.
This autumn, the communications ministry launched
a feasibility project at 11 locations across the country,
in which staff members who support digital utilization
teach mainly elderly people how to operate digital
devices and use administrative and other services
through digital devices.
Repair social division
Nov.11, 2020
Trump's behavior, which lack the qualities and dignity of
a pres ident, has further deepened the confrontation
between his supporters and opponents.
It is obvious that Trump's self-righteous policymaking
process has led to a decline in the functioning of
the bureaucracy and Congress, deteriorating the quality
of U.S. politics.
Biden's victory may have been result of his collecting
ballots cast by voters who expressed their opposition
to Trump's approach.
It must be noted that Biden's policies did not necessarily
win broad support.
Biden said in his victory speech, "I pledge to be
a president who seeks not to divide, but to unify,
urging the public to cooperate.
But it will not be easy to spread the message of
reconciliation among the more than 70 million voters
who cast their votes in favor of Trump.
Structural problems lie behind the growing divisions
in society and a sense of stagnation in the United States.
Only a limited number of sectors have benefited from
the progress of the global economy, free trade and
information technolog in industries, which has made
many people feel that the gap has rather widened.
White workers in industrial and farming areas, among
others, became Trump's rock-solid supporters because
they felt abandoned by politics for the elite.
The "American Dream," the idea that people can win
success by hard work under equal opportunity, is
a fading value.
Making efforts to correct excessive disparities and
expand the number of middle-income earners will be
the first step toward repairing the division of the nation.
Secure and train human resources
in information technology
Nov.10, 2020
In order to catch up in the delayed digitization that has
been exposed by the spread of the novel coronavirus,
there is an urgent task to secure and train human resources
in information technology.
The public and private sectors must work together
to strengthen their efforts.
This year's white paper on the economy and pubic finance
provided a detailed analysis of the impact of the novel
coronavirus pandemic on the economy and the issues that
must be overcome.
The white paper focuses on the uneven distribution of
human resources as a factor that has hindered progress
in digitization.
The white paper pointed out that about 70% of IT-related
human resources who work on systems design,
information processing and other services are concentrated
in the tech industry in Japan.
In the United States, the percentage stops short of less
than 40% and such human resources work in a wide range
of fields such as the finance, services and manufacturing
industries, according to the white paper.
This is certainly because corporate executives outside of
the IT industry in Japan have been slow to recognize
the importance of such personnel and have not actively
recruited them.
Companies without in-house experts tend to be
at the mercy of the tech companies they are out-sourcing
the building of computer systems to, resulting in inefficient
and user-unfriendly products.
Having in-house experts makes it easier to develop and
operate superior systems that are useful to a company's
business.
In recent years, the competition to secure tech-savvy
human resources has become fierce, and tech companies
such as NEC Corp. and Fujitsu Ltd. are beginning to offer
high remuneration based on ability, regardless of age.
Other industries should reconsider strategies to secure
such human resources.
U.S. budget deficit topped
a record $3 trillion for 2020 fiscal year.
November 1, 2020
The federal budget deficit was $3.1 trillion for the 2020 fiscal year,
the Congressional Budget Office estimated on Thursday.
The deficit is a record for the United States in terms of total dollars
and is a direct result of the federal response to the coronavirus
pandemic.
Driving the spike in borrowing were a drop in tax revenues
as the economy contracted this spring amid the pandemic recession
and a surge in government spending to reinvigorate growth,
including enhanced benefits for unemployed workers and widespread
aid to large and small businesses.
Federal spending from April through the end of September was
$4.2 trillion, nearly double the same period in 2019, the budget office
reported.
Individual and corporate income tax receipts fell by $191 billion, or
about 17 percent, in April through September, compared with the year
before.
The 2020 fiscal year concluded at the end of September.
The official budget deficit figures will be released by the Treasury
Department later this month. The budget office estimates are
based on daily figures released by the department.

Quickly present blueprint for
digitization of public administration
September 28,2020
It is important to digitize public administration and
make it more user-friendly for the people.
The government should clearly present its outlook
for the future and a road map.
Prime Minister Yoshihide Suga has announced
his administration's intention to establish a digital
agency as one of its key policies.
The government plans to submit a related bill
to the ordinary Diet session next year.
Within the government, an idea has surfaced
to merge the Cabinet Secretariat's IT General
Strategy Office and related sections of the Economy,
Trade and Industry Ministry and the Internal Affairs
and Communications Ministry.
Unifying digital policies that have straddled multiple
ministries and agencies to establish a structure
to promote digitization is highly significant.
It must be tackled in a speedy manner.
What should be taken up first is digitizing
the administrative services of central government
ministries and agencies and local governments.
In measures to respond to novel coronavirus infections,
confusion arose over the online application for the cash
benefit of \100,000 per person.
The sharing of information on the infectious disease
between the central and local governments stalled,
and schools were notoriously unprepared, such that
they were unable to offer remote classes.
Teleconferences between ministries and agencies
did not go as smoothly as had been intended.
Such incidents occurred because each ministry and
agency has its own computer system and there is
no government-wide strategy.
The digital agency is envisioned as serving
as a control tower to improve such situations.
Keep public documents to leave
infection-control process to history
March 24,2020
How was the threat of a new infectious disease dealt with?
It is the government's responsibility to keep records
of its decision-making process.
Regarding the responses to the outbreak of the new
coronavirus, the government, under guidelines
for managing administrative documents, has
designated the ongoing situation as a historic emergency.
This has become the first case since a guideline policy
on historic emergencies was introduced in 2012.
The latest designation made it mandatory to create
documents such as minutes of important meetings
related to the situation.
The spread of the unknown virus poses a national crisis,
thus it is natural for the government to take a special
approach in managing official documents.
The policy on historic emergencies was introduced
after reflecting on the responses to the Great East
Japan Earthquake.
The administration of the Democratic Party of
Japan failed to compile minutes of meetings
at 10 organizations, including the disaster emergency
response headquarters.
This caused problems in verifying the responses
to the accident at Tokyo Electric Power Co.'s
Fukushima No. 1 nuclear power plant, among other
measures.
In dealing with the infectious disease, Prime Minister
Shinzo Abe's Cabinet must not repeat the same
mistakes.
In the absence of therapeutic drugs or vaccines,
the government is pressed to grope for ways
to prevent the spread of the disease.
The government has taken measures that are
having a major impact on the lives of the people,
such as requesting elementary, junior high and
high schools to close all at once and asking
organizers to refrain from holding events.
It is also taking steps to help children's guardians
and companies who are taking on burdens.
Each ministry and agency on the front line
should keep documents of the details of their
decision-making processes on various measures.
Was the government's judgment appropriate
for each occasion?
Was information smoothly provided to the public?
Each case will be verified afterward and passed
down to future generations.
This accumulation will help in the event of another
similar disaster hitting the nation.
Prepare for prolonged battle
to combat spread of new coronavirus
It can now be seen that countermeasures against
infections with the new coronavirus will need
to be tackled over a long period.
Measures to curb the spread of the disease
must be continued.
At a recent meeting of the government's headquarters
for combating the COVID-19 outbreak,
Prime Minister Shinzo Abe expressed his intention
to thoroughly implement measures against
the infectious disease and called on organizers
to remain cautious about holding large-scale events,
from the viewpoint of preventing infection.
The virus has spread around the world, and there
have been cases in which people returning from Europe
and other parts of the world tested positive.
The risk of the virus entering Japan from abroad
is increasing.
A government panel of experts has analyzed
the current situation in Japan and said the number of
infected people is increasing in some areas and
thus there is a possibility of so-called overshooting,
or an ex-plosive increase in the number of infections.
The prime minister's judgment is believed to have
been based on this severe situation.
Education, Culture, Sports, Science and Technology
Minister Koichi Hagiuda said the government plans
not to extend its initial request for simultaneous
school closures until the regular spring break.
The minister will soon release guidelines that
include the government's basic ideas on reopening
schools in the new semester, among
other measures.
While taking measures to prevent further infection,
it is also essential to carefully look for ways
to get back to normal.
Nations of world must work together
to halt new coronavirus pandemic
March 16, 2020
The World Health Organization (WHO) has recognized
the spread of the new coronavirus as a pandemic -
a worldwide outbreak of an infectious disease.
Countries around the world should cooperate to prevent
the spread of infection.
Infections with the new coronavirus emerged in China
and spread to South Korea and Japan.
Since then, the disease has moved to Italy and other
parts of Europe, Iran and the United States, with over
120,000 people infected in more than 100 countries
and regions.
When a new strain of influenza spread in 2009, the WHO
declared a pandemic at an early stage, but was criticized
for fueling panic as a result.
This may be the reason why it was cautious about
declaring a pandemic this time.
Recognizing a pandemic does not impose any obligation
on countries.
However, those that have infected patients need to
take it as a message urging them to reinforce measures.
The WHO also pointed out that they should continue
their efforts to contain the virus rather than giving up.
It is important for each countryto minimize the number
of victims as much as possible by curbing the speed
of the infection's spread and maintaining medical systems.
Following the WHO's pronouncement, U.S. President
Donald Trump announced a rare measure to ban
travel from Europe, excluding Britain, for 30 days.
From the viewpoint of strengthening the epidemic
prevention system, it is unavoidable that more and
more countries are taking similar measures as
the infection spreads.

Mr.Yoshino's Speech
Industry-academia alliance leads to big payoff
Dec.10, 2019
In order to promote research and development of technologies such as
lithium-ion batteries, that will bring about drastic changes in society,
it is essential to strengthen industry-academia collaboration to put
the results of basic research into practice, and enhance support
for young researchers who will lead the next generation.
“The discovery of a new material called polyacetylene led to lithium-ion
batteries,” Yoshino said on Nov. 11 after meeting Prime Minister Shinzo Abe
at the Prime Minister’s Office to report his win.
Polyacetylene, a conductive plastic, was discovered in the 1970s
by Hideki Shirakawa, 83, a professor emeritus at the University of Tsukuba
and winner of the 2000 Nobel Prize in Chemistry.
Yoshino, then a researcher at Asahi Kasei, took note of polyacetylene and
used it as a material for a prototype of a lithium-ion battery.
This experience led to the practical application of lithium-ion batteries
using carbon materials.
The invention of lithium-ion batteries was not a joint development
between a university and a company. However, based on his experience of
using the outcomes of university research as a starting point,
Yoshino stresses the importance of the industrial world and universities
sharing the “seeds” of new technologies.
Yoshino is currently the president of LIBTEC (Lithium Ion Battery Technology
and Evaluation Center) based in Ikeda, Osaka Prefecture, a technology
research consortium formed of companies and a national research institute.
The consortium is working with the Tokyo Institute of Technology,
Nagoya University and other universities to develop solid-state batteries
that replace liquid electrolytes with solid electrolytes.
A total of about ¥10 billion from state funding is to be invested for five
years
from fiscal 2018 as a project commissioned by the New Energy and Industrial
Technology Development Organization (NEDO).
Solid-state batteries are highly efficient and have no risk of ignition.
If used in electric vehicles, the range of travel will be greatly increased
and accident damage will be reduced. For this reason, solid-state batteries
are considered one of the most promising types of next-generation batteries.
“It is necessary for industry and academia to make all-out efforts to prevent
future markets from being taken over by China, the United States and Europe,”
said Yasuo Ishiguro, managing director of the consortium.
Compensating for weak points
In Japan, some university researchers are reluctant to cooperate
with companies because they are concerned that their research subjects
may be restricted. However, since they were incorporated in 2004,
national universities have been forced to obtain outside funding because
of a decrease of more than ¥140 billion in subsidies allocated to them
by the central government.
Companies are also losing the capacity to conduct basic research
due to cost reductions and intensifying competition over development.
Industry-academia collaboration is a means of compensating
for each other’s weaknesses.
Healsio, Sharp Corp.’s hit product known as the “oven that bakes
with water,” was created in cooperation with Osaka Prefecture University,
which engaged in research on the decomposition of extremely toxic dioxin
by high-temperature steam.
Kyoto University, Denso Corp. and other entities are jointly researching ways
to reduce the weight of automobiles by using minutely fiberized wood
for things like car parts. The technology is expected to be put into practical
use in a few years.
African swine fever
Another wild boar found dead near the demilitarized
zone tested positive for African swine fever on Oct.16
in South Korea.
It is the seventh dead boar confirmed to have
the virus since the outbreak of ASF began last month.
The S.Korea Environment Ministry said that the infected
carcass was found just 1.4 km from a site in Cheorwon,
Gangwon Province where two other dead boars
with the virus were found last Saturday.
In a bid to contain the disease, which is deadly
to swine, fences were set up near the DMZ
to prevent wild boars moving to other areas.
S.Korean soldiers have been sent since Tuesday
to cull all wild boars around the DMZ and
killed 57 in a day.
According to the Ministry of Agriculture, Food and
Rural Affairs, the number of confirmed ASF cases
at pig farms stands at 14.
No new cases have been reported
since the last one was confirmed on Oct. 9.
African swine fever in S.E. Asia
gives U.S. crop exporters chills
October 17, 2019
These hard-won gains are now at risk
lof being reversed by African swine fever:
Since Southeast Asia accounts for 15 percent of
total corn and soymeal imports, any stbstantial
downturn in animal numbers will lead to
a commensurate drop in feed crop imports.
According to two traders who sell U.S. and South
American crops into the region, corn and soymeal
purchases are expected to drop by roughly
1 million tons each this year in Vietnam alone.
The traders declined to be identified because
they are not authorized to speak to media.
In Vietnam, the world's sixth-largest pork producer,
about 4.7 million pigs have already been culled
as African swine fever has spread to all 63 of
the country's provinces since being detected
in February.
By end-July, the country's pig herd had shrunk
by 18.5 percent to 22.2 million animals.
"In my village, there are a lot of pigs infected
with swine fever," said Nguyen Thi Hai, a pig farmer
at Tu Nhien village, outside Hanoi, in business
since 2006. "At least, more than half of them
were infected and killed."
Meanwhile Thailand - the world No. 5 soymeal
importer - this month began culling pigs amid
heightened fears of a potential outbreak of
African swine fever after the death of two hogs
without explanation in a province close to
swine fever-hit Myanmar earlier this month.
For now, industry watchers say Asia's farmers
and U.S. grain exporters will need to strap
in for a bumpy ride.
"It has been a big blow to feed demand as China
has culled more than 30 percent of its herd," said
Ole Houe, director of advisory services at brokerage
IKON Commodities in Sydney.
"Now it is spreading into Vietnam, the Philippines
and South Korea.
It is more bad news for feed grains growers
in America and livestock farmers in Asia."

Why did Typhoon19 Hagibis
shatter Japan's rainfall records?
October 16, 2019
Typhoon Hagibis, one of the strongest in Japan's history,
left a trail of destruction in the center and the north of the country.
It dumped unprecedented amounts of rain over a wide area,
forcing authorities to issue emergency warnings at the highest level
possible for 13 prefectures.
The alert covered nearly one third of the entire country.
How much rain?
So, how much rain did Hagibis bring?
The bars in the graphic below indicate the total rainfall.
Over 100 observation spots, from the Kii Peninsula all the way
up to the Tohoku region, experienced unprecedented rainfall.
Hakone, which is known for its hot springs, experienced the heaviest.
The area received over a meter of rain -- three times more
than the typical October.
Most of the water came down in a short period of time,
with 922.5mm falling in 24 hours, setting a new national record.
Why was the rain so bad?
Typhoons in general produce huge amounts of rain as
they bring moisture from the tropics.
That's why the Pacific side of Japan, where typhoons frequently hit,
see much more rainfall than the opposite side of the country.
However, even compared to other typhoons,
Hagibis dumped extraordinary amounts of water.
What made Hagibis so extreme?
Three factors were involved:
1. Intensity
Hagibis was one of the strongest tropical systems on earth this year.
It underwent a rapid intensification, resulting in its central pressure
dropping from 992hPa to 915hPa in 24 hours.
This is the ninth-biggest pressure drop ever recorded in a typhoon.
The intensification was caused by the warm waters off the Mariana Islands.
This ocean area is infamous among meteorologists, as past typhoons
that developed in the area frequently caused significant damage in Japan.
The area is far from any land masses and the water is warm,
so there is almost nothing to weaken the storms.
In addition, the sea surface temperature over the area was about
2 degrees Celsius warmer than normal. That fed more moisture
into the typhoon, making Hagibis an even more significant rain maker.
2. Speed
Hagibis was a slow-moving typhoon with a forward velocity of only about
30kph.
Fall typhoons tend to move faster as the storms interact with the jet stream
over the country.
But the jet stream was present to the north this time, making Hagibis move
slowly.
3. Size
On top of the speed, Hagibis was categorized as a large typhoon,
as the area of tropical-force winds was over 1,400km in diameter.
Torrential rain started to fall more than a day before it reached land.
With the large size and slow movement, moist winds continued to flow in.
That led to record-breaking rainfall in many places.
Super typhoons on the rise
Experts say the frequency with which we see storms as strong
as super typhoons will increase in the near future.
The ocean is expected to heat up by 2 to 3 degrees Celsius by the end
of this century, and that will allow powerful storms to form more frequently
over the northwestern Pacific. Some say super storms with pressures of
860hPa and gusts of up to 320kph are possible.
When it comes to typhoons, Japan is one of the best-prepared countries
in the world, but we may have to reconsider our countermeasures,
both in terms of infrastructure and preparation.
Spread of Trump-style politics
is eroding multilateral cooperation
The Yomiuri Shimbun, Sept. 26, 2019
International cooperation is crucial for coordinating
the interests of each country and maintaining
a stable world order.
It is important to continue advocating the significance
of this approach and prevent the spread of excessive
"country first" policies.
In a speech at the United Nations, U.S. President
Donald Trump declared:
"The future does not belong to globalists.
The future belongs to patriots."
Trump also said,
"Wise leaders always put the good of their own
people and their own country first."
Of course, every country's leader gives priority
to the interests of one's own nation.
However, terrorism, climate change, poverty and
other issues that transcend national boundaries
cannot be resolved if each country acts alone.
It is obvious that pursuing only the gains of one's
own country will cause conflicts to spread and also
harm wider economic interests.
Trump's style of politics is already starting to spread.
Leaders of Hungary, Turkey, Brazil and other nations
have gained support by claiming internationalism is
an idea orchestrated by the elite that disregards
national interests.
The elements of populism are plain to see.
North Korean Missile Comes
with Angry Message
to South Korea’s President Moon
When North Korea said on Friday that it had tested a new, more advanced
missile,
it pointed the finger of blame at one man: Moon Jae-in, the South Korean
president,
who just last year embraced Kim Jong-un at their countries’ border.
The North said Mr. Kim, its leader, had personally arranged the missile test on Thursday
to counter what it called Mr. Moon’s “double-dealing”: talking peace with
North Korea
even as he bought state-of-the-art F-35 stealth jets and planned joint
military drills
with the United States.
Some analysts said Mr. Kim, in singling out Mr. Moon, was venting anger over his failure
to win relief from crippling economic sanctions over his nuclear program,
with talks
between his government and the United States having stalled.
On the day the North made its announcement, bad economic news arrived:
South Korea’s central bank said the North’s economy had shrunk by 4.1 percent
last year, its worst contraction since 1997.
“Kim Jong-un is clearly frustrated,” said Ko Yu-hwan, a professor of North Korean
studies at Dongguk University in Seoul, the South’s capital. “He had hoped
that
President Moon would be able to help persuade Washington to ease sanctions.
He now seems to have concluded that South Korea is really at Washington’s beck
and call.”
Typhoon Faxai Affects
2.7 M. Train Passengers
in Tokyo Area
Jiji Press, Monday, September 9, 2019
Typhoon Faxai forced East Japan Railway Co.
to halt 3,837 non-Shinkansen trains in the Tokyo
area Monday, affecting some 2,777,000 people,
the company said.
The railway operator, known as JR East, said Sunday
that it would halt Monday's non-Shinkansen train
services until around 8 a.m. due to the typhoon.
But the resumption was delayed on many train lines
due to the typhoon moving at a slower speed than
anticipated, damage to rail equipment and
the discovery of objects such as fallen trees on rail tracks.
Some sections, including on the Yokosuka Line, were
unable to resume services on Monday.
The company restricted entry of passengers into some
stations such as Ofuna on the Tokaido Line, forcing
many people to wait for a long period of time to get
on trains.
Seoul, Busan pass the bills
to boycott Japanese goods
Jiji Press, Sunday, September 8, 2019
In a fresh development illustrating growing tensions
between Japan and South Korea, the Seoul city
assembly enacted Friday an ordinance calling
on the city government of the South Korean capital
to restrict its purchases of products of certain
Japanese companies.
The city assembly of Busan passed a similar ordinance
the same day.
Seoul and Busan are the most and second-most
populated cities in South Korea, respectively.
Calls for introducing ordinances for a boycott of
Japanese goods are growing in other South Korean
municipalities as well.
The Seoul and Busan ordinances branded Japanese
businesses that requisitioned people from the Korean
Peninsula to work for them during Japan's colonization
of the peninsula from 1910 to 1945 and exploited or
inflicted damage on these people as "war-criminal
companies."
Designated as such companies in the Seoul ordinance
are more than 280 businesses, including Mitsubishi
Heavy Industries Ltd. and Nippon Steel Corp.
The ordinance claimed that no official apology or
compensation has been made although these companies
inflicted damage on South Korean people such as through
forcible requisition of workers.
The majority of members of the Seoul and Busan
assemblies have close ties with the administration of
South Korean President Moon Jae-in.
They apparently hope to support the administration
by leading the boycott campaign and enhancing
anti-Japan sentiments across South Korea.
The moves are likely to affect exchanges between
local communities of Japan and South Korea.
Relations between the two countries have been
frosty due to wartime labor and other issues.
In Tokyo, Chief Cabinet Secretary
Yoshihide Suga said at a press conference Friday
that the latest moves by the Seoul and Busan
assemblies are "extremely regrettable because
the ordinances accuse certain companies of
our country unfairly based on improper and
unreasonable claims, and can bring economic
disadvantages."
Behind the spreading boycott campaign is a backlash
against Tokyo's tightened controls on exports to South
Korea, including the removal late last month of
the neighboring country from its list of trusted trading
partners qualified for preferential treatment in export
procedures.


1 killed, at least 30 injured
in Yokohama derailment
Friday, September 5, 2019
One person was killed and at least 30 injured
when the first three cars of an express train
on the Keikyu Line derailed after the train collided
with a truck at a crossing in Kanagawa Ward,
Yokohama, at around 11:40 a.m. Thursday.
The person killed was believed to be the 67-year-old
male driver of the truck.
Black smoke and flames were seen at the crossing,
which is located between Kanagawa-Shinmachi
Station and Nakakido Station on the Keikyu Line.
According to the Kanagawa prefectural police and
Keikyu Corp., the train departed from Aoto Station
bound for Misakiguchi Station.
It collided with the truck, which entered the crossing
from the train’s right side just after the train
passed Kanagawa-Shinmachi Station. The train's
next scheduled stop was Yokohama Station.

Opponents of 'no-deal' Brexit
defeat Johnson
LONDON, Wednesday, September 4, 2019
British lawmakers defeated Boris Johnson in Parliament
on Tuesday in a bid to prevent him taking Britain out of
the European Union without a divorce agreement,
prompting the prime minister to announce that
he would immediately push for a snap election.
The government was defeated by 328 to 301
on a motion put forward by opposition parties and
rebel lawmakers in Johnson's party - who had been
warned they would be kicked out of the Conservative
Party if they defied the government.
More than three years after the United Kingdom voted
in a referendum to leave the EU, the defeat leaves
the course of Brexit unresolved, with possible
outcomes still ranging from a turbulent "no-deal" exit
to abandoning the whole endeavor.
Tuesday's victory is only the first hurdle for lawmakers,
enabling them to take control of parliamentary business.
On Wednesday they will seek to pass a law forcing
Johnson to ask the EU to delay Brexit -for a third time
- until Jan. 31 unless he has a deal approved by
Parliament beforehand on the terms and manner of
the exit.
It puts Johnson in a similar bind to that faced
by his predecessor Theresa May, who failed three times
to get the backing of lawmakers for the Withdrawal
Agreement that she had negotiated with the EU.
Johnson took over from her six weeks ago with a promise
that his more robust approach would force a better deal
out of the EU that would satisfy Parliament.
The 21 Conservative rebels who now face expulsion
from the party include Nicholas Soames, the grandson
of Britain's World War II leader Winston Churchill, and
two former finance ministers - Philip Hammond and
Kenneth Clarke.
"I don't want an election, but if MPs vote to stop
negotiations and compel another pointless delay
to Brexit, potentially for years, then that would be
the only way to resolve this," Johnson told Parliament
after the vote.
"I can confirm that we are tonight tabling a motion
under the Fixed Term Parliament Act."
China lodges tariff case
against U.S. at WTO
China has lodged a complaint against the United States
at the World Trade Organization over U.S. import duties,
the Chinese Commerce Ministry said on Monday.
The United States began imposing 15% tariffs
on a variety of Chinese goods on Sunday and
China began imposing new duties on U.S. crude oil,
the latest escalation in their trade war.
China did not release details of its legal case but said
the U.S. tariffs affected $300 billion of Chinese exports.
The latest tariff actions violated the consensus reached
by leaders of China and the United States in a meeting
in Osaka, the Commerce Ministry said in the statement.
China will defend its legal rights in accordance
with WTO rules, it said.
The lawsuit is the third Beijing has brought to challenge
U.S. President Donald Trump's China-specific tariffs
at the WTO, the international organization that limits
the tariffs each country is allowed to charge.
U.S. officials say that they are penalizing China
for theft of intellectual property that is not covered
by WTO rules, although many trade experts say that
any tariff hike above the allowed maximum must be
justified at the WTO.
Many experts also decry China's decision to fight fire
with fire, by imposing tariffs on U.S. goods imported
into China, also without the WTO's approval.
On Friday the United States published a written defense
in the first of the three legal cases, asserting that
China and the United States agreed the issue
should not be judged at the WTO.
"China has taken the unilateral decision to adopt
aggressive industrial policy measures to steal or
otherwise unfairly acquire the technology of its trading
partners; the United States has adopted tariff measures
to try to obtain the elimination of China's unfair
and distortive technology-transfer policies," it said.
China had chosen to respond not by addressing U.S.
concerns but with its own tariffs, "in an effort
to maintain its unfair policies indefinitely".
The U.S. submission also said its actions were exempt
from WTO rules because they were "measures
necessary to protect public morals" - a clause used
in the past to argue for trade restrictions over
gambling, animal rights and public broadcasting.
Under WTO rules, Washington has 60 days to try
to settle the latest dispute.
Then China could ask the WTO to adjudicate,
a process that would take several years. It could end
with China gaining WTO approval to take trade
sanctions, if the United States is found to have broken
the rules.
Trump Heaps More Tariffs
on China, Still No Deal in Sight
Monday, September 2, 2013
The Trump administration slapped tariffs on roughly
$110 billion in Chinese imports on Sunday, marking
the latest escalation in a trade war that's inflicting
damage across the world economy.
The 15% U.S. duty hit consumer goods ranging
from footwear and apparel to home textiles and
certain technology products like the Apple Watch.
A separate batch of about $160 billion in Chinese
goods -- including laptops and cellphones -- will be hit
with 15% tariffs on Dec. 15. President Donald Trump
delayed part of the levies to blunt the impact
on holiday shopping.
Investors sought the safety of the yen, which edged
higher against the dollar as currency markets opened
for trading. The offshore yuan pared some losses
to trade at 7.1682 per dollar Monday at 10:38 a.m.
in Beijing after the PBOC set the fixing rate stronger
than all estimates.
Asian stocks fell with U.S. equity futures after the tariffs
kicked in, even though the measures had been widely
anticipated. S&P 500 futures opened 1% lower before
paring losses, and Treasury contracts advanced.
Trump's 15% tariffs on $112 bil.
in Chinese goods take effect
The Associated Press, Sunday , September 1, 2019
The Trump administration's latest round of tariffs
on Chinese imports took effect early Sunday,
potentially raising prices Americans pay for some
clothes, shoes, sporting goods and other consumer
goods in advance of the holiday shopping season.
The 15 percent taxes apply to about $112 billion
of Chinese imports.
All told, more than two-thirds of the consumer goods
the United States imports from China now face higher
taxes. The administration had largely avoided hitting
consumer items in its earlier rounds of tariff hikes.
But with prices of many retail goods now likely
to rise, the administration's move threatens
the U.S. economy's main driver:
Consumer spending.
As businesses pull back on investment spending and
exports slow in the face of weak global growth,
American shoppers have been a key bright spot
for the economy.

Protesters rally at Hong Kong
airport to disrupt travel
as heavy storm approaches
HONG KONG, Sunday, Sep 1, 2019
Hundreds of protesters gathered outside Hong Kong's
international airport on Sunday, disrupting travel in a bid
to focus global attention on their fight for greater
democracy in a city facing its biggest political crisis
in decades.
Trains to the airport were suspended and traffic was
congested after protesters urged the public to overwhelm
road and rail links to one of the world's busiest airports.
But passengers continued to enter and leave the terminal
freely, and planes were still taking off.
Rain fell as a tropical depression approached Hong Kong
from the southeast.
Authorities raised the number three typhoon signal,
warning of a possible heavy storm on the way.
Police warned the airport gathering was illegal
and said they would soon conduct a “dispersal operation.”
“The police warn all protesters to stop their illegal acts
and leave immediately,” they said in a statement.
Sunday’s demonstration comes after police and
protesters clashed overnight in some of the most intense
violence since unrest erupted more than three months
ago over concerns Beijing was planning to erode
the autonomy granted to the city when it was
handed back to China from Britain in 1997.
China denies the charge of meddling in Hong Kong,
which it says is an internal affair. It has denounced
the protests and warned of the damage to the economy.
Protesters outside the airport, many wearing hard hats
and gas masks, chanted “Fight for freedom! Stand
with Hong Kong!” as riot police watched from inside
the terminal building.
“We plan to disrupt activity at the airport to draw
attention to what the government and the police are
doing to us,” said one 20-year-old protester,
asking not to be named.
“If we disrupt the airport more foreigners will read
the news about Hong Kong.”
Three weeks ago, some flights were delayed or
canceled after protesters swarmed the airport more
foreigners will read the news about Hong Kong.”
Several hundred demonstrators also gathered outside
the British Consulate in central Hong Kong, waving
Union Jack flags and chanting “God save the Queen.”
During a chaotic night of violence that lasted into
the early hours of Sunday, police fired tear gas,
water cannons and rubber bullets and protesters
threw petrol bombs.
As government helicopters hovered overhead,
protesters who had been banned from demonstrating
set fires in the streets and threw bricks at police
near government offices and Chinese military
headquarters.
The Defense Ministry
seeks record ¥5.32 trillion
defense budget
Saturday, August 31, 2019
On Friday, The Defense Ministry requested a record
¥5.32 trillion ($50 billion) budget for fiscal 2020
as it stressed the need to beef up the nation's defense
capabilities in domains such as outer space and
cyberspace.
The sum marks a 1.2 percent rise from the initial budget
for the current fiscal year through March 2020.
If approved, the defense budget will grow for the eighth
year in a row under Prime Minister Shinzo Abe.
The ministry asked for ¥52.4 billion to strengthen
its capabilities in space amid the intensifying race
among major powers such as the United States,
Russia and China to develop technologies in the domain.
A space operation unit will be newly formed inside
the Air Self-Defense Force, including purchases of
equipment to detect electromagnetic interference
with Japanese satellites, as well as an optical telescope
to monitor space debris and unidentified objects
in outer space.
The ministry is seeking ¥23.8 billion to expand staffing
levels for the cyberdefense unit and take other
measures for cybersecurity.
A budget of ¥20.7 billion will be earmarked
to develop a “stand-off electronic warfare aircraft,”
which can hinder invading enemy forces by jamming
equipment.
These requested items reflect the government's latest
national defense guidelines adopted last December,
which said that the fields of cyberspace, outer space
and electronic warfare have the potential to
"fundamentally change the shape of national security"
that has so far mainly focused on conventional ground,
sea and air domains.
As for the planned deployment of the U.S.-developed
Aegis Ashore land-based missile defense batteries,
the ministry asked for ¥12.2 billion to acquire
the vertical launching system, develop human
resources and conduct surveys to evaluate candidate
sites for its deployment .
To counter the North Korean threat, the ministry has
sought to introduce the Aegis Ashore system
in Akita and Yamaguchi prefectures.
But the plan hit a roadblock when it was disclosed
in May that a geographical survey for the deployment
used erroneous data, drawing harsh local backlash.
It also requested ¥3.1 billion to start upgrade work
on the Izumo flat-top helicopter carrier to enable it
to transport and launch fighter jets, a plan specified
in the defense guidelines.
About ¥84.6 billion is eyed to buy six F-35B fighter jets
to use on the Izumo. They are capable of short
takeoffs and vertical landings.
The ministry said in the budgetary request that
it is also seeking funds to develop fighters to succeed
the ASDF's F-2s, which it estimates will be retired
in the 2030s, without specifying a figure.


Hundreds of thousands ordered
to evacuate after heavy rain
hits Northern Kyushu
Thursday, August 29, 2019
Hundreds of thousands of people in Northan Kyushu
have been ordered to evacuate their homes
as torrential rain continues to hit the region.
Authorities issued an emergency alert Wednesday,
warning of mudslides, flooding and swollen rivers
in Fukuoka, Saga and Nagasaki prefectures.
The agency initially issued its highest level alert,
known as a level five, but later downgraded it to
a level four.
A total of 870,000 people living in the area have
been ordered to evacuate, as of 3:30 p.m.,
local time Wednesday.
The heavy rain, which started on Tuesday, has seen
some areas hit by more than 100 mm of rainfall
in an hour, public broadcaster NHK said.
More rainfall is expected throughout Wednesday,
according to the country's Meteorological Agency.
Abe "Africa will be
mega-economic zone"
The Yomiuri Shimbun, August 30, 2019
Prime Minister Shinzo Abe said he intends to help
Japanese companies expand their business and
investment in Africa at a public-private business
dialogue session of the seventh Tokyo International
Conference on African Development (TICAD)
in Yokohama on Thursday.
Japanese and African officials and business leaders
attended the session.
"The scale of the African market continues to grow.
We can see the day is coming when the whole
continent will become a mega-economic zone,"
said Abe.
However, he added, "If investment destinations are
mired in debt, companies will be unable to expand
their business," indicating the government will support
the proper management of public debt.
Egyptian President Abdel-Fattah el-Sissi, who is
cochairing the TICAD conference with Abe, said
that "each government can set a model for
encouraging public-private cooperation
by focusing on strengthening cooperation
with the private sector."
Representatives of the Japan Business Federation
(Keidanren), the Japan Association of Corporate
Executives (Keizai Doyukai) and others also
attended the session.
"We want the public and private sectors to work
together," one representative said.
The session introduced efforts by the Japan Business
Council for Africa, a public-private partnership
established in June to promote investment by
Japanese companies in Africa.
Participants also discussed ways to develop
businesses using advanced technology.
Trump criticized Moon Jane
August 28, 2019
At G7 summit, American President Trump said
"I could not trust Korean President Moon Jane "
and he criticized Moon severely for twice.
While it is in an opening night of G7 summit
meeting opened up in France Trump criticized
and leaders and others do an argument
he said again that "a person called 文在寅 is
untrustworthy, so cut and brought it down.
According to the person concerned with government,
it is said that Trump repeated "Kim Jong-un criticized
to me South's President a person telling a lie" .
Trump poses "why such a person became the President.
at the dinner party held at night of the second day.
Danes disgusted
after Trump cancels visit
over Greenland
August 22, 2019
Trump's decision to cancel his state trip to Denmark
after being told that Greenland is not for sale has caused
a mix of anger and confusion among current and former
Danish politicians.
Trump's decision "came as a surprise," the Royal
House's communications director told Denmark's
public broadcaster. "That's all we have to say about that."
Former Prime Minister Helle Thorning-Schmidt was
more explicit in her criticism:
"Is this some sort of joke? Deeply insulting
to the people of Greenland and Denmark."
Danish Prime Minister Mette Frederiksen told
Danish media Sunday that Trump's reported desire
to purchase Greenland is an "an absurd discussion"
and that the territory is not for sale."
Exports keep falling amid U.S.-China row
Jiji Press, Tuesday, August 20, 2019
Exports dropped for the eighth straight month
in July mainly due to negative effects of
the U.S.-China trade row, Ministry of Finance
data showed Monday.
As a result, Japan logged a customs-cleared
trade deficit of \249.6 billion, up 9.8 percent
from a year before.
The latest red ink was bigger than the median
forecast of \200 billion in deficit in a Jiji Press
survey of 18 research institutes.
Exports fell 1.6 percent to \6.64 trillion, and
imports slid 1.2 percent to \6.89 trillion,
the ministry said in a preliminary report.
Overall imports were pulled down by a fall
in imports of petroleum products from
the United Arab Emirates and of crude oil
from Iran.
In trade with mainland China, Japanese exports
dived 9.3 percent to \1.23 trillion for a fifth
straight decline, reflecting stagnant exports of
automotive parts and semiconductor-
manufacturing equipment.
The sluggishness may have resulted from
a mild slowdown in the Chinese economy,
a ministry official said.
Meanwhile, imports advanced 2.8 percent
to \1.61 trillion.
As a result, Japan posted a deficit of \383.8
billion with mainland China, up 79.9 percent.
With South Korea, Japan's exports sank 6.9
percent to \436.3 billion, and imports dropped
8.6 percent to \275.7 billion.
The main reason for the drops was a slowdown
in the global economy.
But soured relations between Tokyo and Seoul,
partly stemming from Japan's tightened
controls on semiconductor material exports
to South Korea, may have also weighed on trade.


Hong Kong Protesters Continue
Demonstration Despite Rain
Monday, August 19, 2019
Hundreds of thousands of Hongkongers have taken
to the streets to protest a controversial extradition bill
that could send Hong Kong residents to mainland
China to be tried in court.
Hong Kong's Chief Executive, Carrie Lam, says
the bill is meant to prevent Hong Kong from becoming
a safe haven for fugitives.
But its opponents fear that Hong Kong would be exposed
to China's flawed judicial system, which would lead
to further erasure of the city's judicial independence.
At first Carrie Lam was determined to move forward
with the bill. But after a series of massive protests,
she announced she would “indefinitely suspend”
the bill.
But protesters aren't accepting the suspension, and
have started demanding its complete withdrawal.
They've also begun calling for Lam's resignation.
But this rise in tensions is about a lot more than a bill.
To understand why this bill hits a nerve with Hongkongers,
it's important to understand Hong Kong's relationship
with China - and exactly how the bill would tip the scales
in China's favor.
Life-threatening heat wave to strike Japan on Aug. 17, JMA warns
August 17, 2019 (Mainichi Japan)
TOKYO -- A life-threatening heat wave is expected
to hit western and eastern Japan on Aug. 17
due to a strengthening high-pressure system
over the Pacific Ocean, the Japan Meteorological
Agency (JMA) warns.
The temperature is expected to surpass 35 degrees
Celsius in the Chugoku and Shikoku regions in western
Japan, and the Kanto region around Tokyo.
The temperature is forecasted to hit 38 C in Saitama
Prefecture north of Tokyo, 37 C in Kanto's Ibaraki,
Gunma and Yamanashi prefectures, and 36 C
in Tochigi Prefecture and Tokyo, as well as in Kyoto
Prefecture in Japan's west.
"The high temperatures will pose an increasing threat
to people's lives. We'd like you to frequently drink
water and take salt, and be careful even when you
are indoors," a JMA official said.
Japan Post Group's corporate
governance seriously deficient
Yomiuri, August 5, 2019
The lack of corporate governance is too dreadful
to watch, with respect to the latest case of
inappropriate contract-winning.
All possible measures must be taken to uncover
the whole truth behind the problem while also aiding
customers who have suffered losses.
The issue concerns the discovery of a large number
of contracts inappropriately drawn up by Japan
Post Insurance Co., a corporation under the umbrella
of Japan Post Holdings Co.
The number of questionable cases has reached 183,000.
Investigations have been made into contracts signed
over the past five years, and their results show that
the total number of dubious cases is double
an initial figure of 93,000.
The new figure includes a noticeable number of
particularly heinous cases, including ones in which
employees used elderly persons' trust in post offices
to their own advantage by making the elderly sign
unnecessary contracts to fulfill the quotas set for
the employees.
Their inclination to treat customers so cavalierly
cannot be overlooked.
Japan Post Holdings' earnings structure is such that
its poor performance in the mail-delivery business
is made up for by profits gained by its financial arms,
Japan Post Insurance and Japan Post Bank Co.
One wonders whether the holding company may have
coerced employees in charge of sales to increase
their sales through improper means while promoting
the privatization of its operations.
Japan Post Holdings President Masatsugu Nagato
and other top officials must bear a heavy responsibility
in this respect.
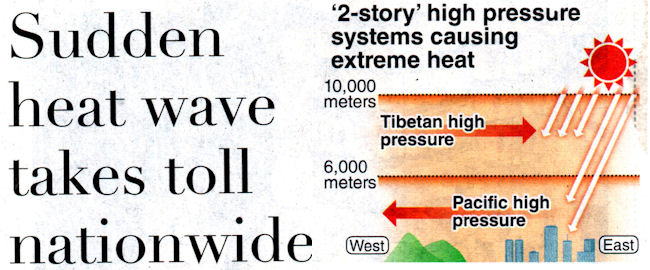
August 5, 2019
The Japanese archipelago has been suffering under
extreme heat since the end of the rainy season
in late July, which has led to a spike in deaths and
people being taken to the hospital due to heatstroke.
This is believed to be partly because bodies that were
accustomed to the cool rainy season have been unable
to adapt to the sudden change in temperature.
Triple the casualties
A 28-year-old man dressed in a costume at the Hirakata
Park amusement facility in Hirakata, Osaka Prefecture,
died of heatstroke while rehearsing a show on the evening
of July 28, four days after the end of the rainy season
in the Kinki region.
Temperatures in the city reached 33.2 C that day.
There has been a nationwide increase in the number
of people collapsing due to heatstroke.
According to the Tokyo Fire Department, the number of
people taken to the hospital per day in July in Tokyo,
excluding Inagi and the islands, was in the single digits
up to July 17.
However, the figure soared to more than 100 on July 29,
the day the rainy season was declared over in the Kanto
and Koshin regions.
The numbers have continued to grow, with 333 people
being taken to the hospital on Thursday and 341 people
on Friday.
Fire and Disaster Management Agency statistics show
that only one person died of heatstroke from July 1
to 23, but deaths have since risen rapidly, with 11
occurring in the five days from July 24.
Target? Of course JAPAN !
N. Korea Fires Multiple Projectiles
Seoul, Aug. 2, 2019, Jiji Press
North Korea launched projectiles twice toward the Sea of Japan
from a point around Yonghung in the eastern part of the country early Friday,
according to the South Korean military's Joint Chiefs of Staff.
The launches were carried out around 2:59 a.m. (5:59 Thursday GMT) and
around 3:23 a.m.
An official of the South Korean military said that it is keeping a close
watch
on the situation and remains prepared to respond to more launches.
North Korean leader Kim Jong Un has been demanding that the United States
and South Korea cancel their joint military exercise slated to start on
Monday.
The latest launches, the third such action by North Korea in less than 10 days,
may have been intended to show Pyongyang's frustration at the planned drill
and
demonstrate its confrontational posture, analysts said.

The Japan Times, July 31, 2019
North Korea fired multiple unidentified “projectiles” off its east coast,
the South Korean military said Wednesday, less than a week
after launching two new types of missiles from the same area.
The projectiles were launched from the Hodo Peninsula, in South Hamgyong
Province, the South Korean military’s Joint Chiefs of Staff said in a statement,
adding that it was monitoring the situation in the event of additional
launches.
The projectiles did not land in Japan’s territorial waters or exclusive
economic
zone, the Japanese Defense Ministry said in a statement.
The U.S. also said it was following the reports, but did not immediately confirm
the launches.
“We are aware of reports of a missile launch from North Korea and we will
continue
to monitor the situation,” a State Department official told The Japan Times.
The apparent test-firings come less than a week after Pyongyang lobbed
what
Japan said on Monday were two short-range ballistic missiles into the Sea
of Japan.
The move came after a North Korean official told the White House last week
that
working-level talks to revive denuclearization negotiations with Pyongyang
would begin
very soon, Reuters quoted a senior U.S. administration official as saying
Tuesday.
The U.S. National Security Council official had been in the region for unrelated talks,
and traveled to the Demilitarized Zone dividing the two Koreas to deliver
some
photographs commemorating the June 30 meeting at the DMZ between U.S. President
Donald Trump and North Korean leader Kim Jong Un, the U.S. official said.
Denuclearization talks between Washington and Pyongyang have been stalled since
Trump met Kim Jong Un in Hanoi at the end of February. Those talks collapsed
amid major differences over the scope of Pyongyang’s denuclearization and
potential sanctions relief by Washington.
The White House had said it hopes the long-stalled working-level talks with the North
would restart sometime this month after the DMZ meeting injected fresh
momentum
into the negotiations.
The North’s recent spate of weapons tests, including its launch of two
“new-type”
missiles last week and a similar launch in May has been seen as an attempt
by Pyongyang to gain leverage and heap pressure on both Seoul and Washington
ahead of any rebooted talks.
Trump has downplayed the recent launches and expressed an interest in reviving
the talks, and sent the U.S. special envoy to North Korea, Stephen Biegun,
to accompany Secretary of State Mike Pompeo to Bangkok for meetings
on the sidelines of the Association of Southeast Asian Nations Regional
Forum
this week.
The two sides could lay the groundwork for the talks’ restart during meetings
on the sidelines of that forum.

Rainy season ends
in Japan's Kanto-Koshin region,
giving way to intense heat
NationalNews, Tuesday, Jul 29, 2019
The annual milestone came 30 days later than last year,
and temperatures will likely start rising to dangerous highs.
The rainy season has ended in Tokyo and other areas
in the Kanto-Koshin region of eastern and central Japan
30 days later than last year, the Meteorological Agency
said Monday, giving way to intense heat.
Rainfall between June 7 and July 28 hit 526 milimeters
in Choshi, Chiba Prefecture, 480.5 mm in Maebashi,
Gunma Prefecture, 459 mm in Utsunomiya, Tochigi
Prefecture, and 446 mm in Yokohama.
Now that the rainy season has passed, temperatures
of over 31 degrees are forecast through this weekend
across the nation.
In the cities of Osaka and Fukuoka, temperatures are
expected to exceed 35 degrees.
On Monday the weather agency urged precautions
against heatstroke, advising that people stay hydrated
and find places to cool off.
Last Saturday, a 91-year-old woman died of heatstroke
in Saitama Prefecture after she was found lying
in her garden at around noon.
According to the Fire and Disaster Management Agency,
about 95,000 people were taken to hospitals
for heatstroke during the May-September period
last year, exceeding the previous high of 58,000
logged in 2013.

Unrest has been deepening
in Hong Kong
Monday, July 29, 2019
Hong Kong`s some protesters clashed with police and
took aggressive action on Sunday.
A series of rallies have been held in Hong Kong
over a controversial extradition bill that would allow
the government to transfer suspects to mainland China.
On Sunday, tens of thousands of people held
a demonstration in a downtown park.
They then marched through the streets in protest
against police violence and the forcible removal
of young demonstrators.
A crowd of mainly young people clashed with police
at several locations along the streets to the Chinese
government's liaison office as well as in a commercial
district.
Some youths threw bricks at police officers and
tried to push them back by setting fire to pieces
of cardboard on wheel barrows.
Police used tear gas and rubber bullets to break up
demonstrators. They detained at least 49 people.
The confrontation continued through the early hours
of Monday.
The Hong Kong government says at least 16 people
were injured in the clash.
In a statement, the Hong Kong government condemns
the violence of some demonstrators, saying it led
to the deterioration of social order.
The government says it fully endorses what it sees
as a rigid execution of law by police.
A sense of resentment is growing among Hong Kong
youths against the government and police.
They have already worked out plans to hold weekly
rallies until mid-August.

34died in arson
at animation studio in Kyoto
July 19, 2019
33 people died when a fire, caused by arson,
broke out at a studio by animation production
company Kyoto Animation Co. in Kyoto, on July 18.
According to the fire department, a nearby resident
made an emergency call at about 10:35 a.m.,
saying they had heard the sound of an explosion.
Officials of the prefectural police rushed to the studio
and found a man, 41, on a road near the studio.
The man told them, “I sprinkled liquid on the first floor
and set it on fire.”
Japan, S. Korea Apart in Talks
on Tokyo's Tighter Export Controls
Jiji Press, Friday, July 12, 2019
Working-level officials from Japan and South Korea
failed to narrow gaps in the two countries' first talks
on Friday on Tokyo's tightened controls on exports
of semiconductor materials to South Korea.
During the meeting in Tokyo, Japanese officials
blamed South Korea's poor export controls
for the trade measure, according to a senior
Japanese industry ministry official with access
to the talks.
The Japanese participants insisted that the measure
did not violate World Trade Organization rules and
was not taken for retaliation.
The Japanese officials explained that an inappropriate
case in South Korea, cited by Tokyo as the reason
for the measure, involved bilateral trade,
not shipments to third countries like North Korea.
Tokyo, which regarded Friday's meeting as nothing
but an occasion to confirm facts, refused to hold
any talks on the retraction of the trade measure.
The “tsuyu” rainy season
started in the Kanto-Koshin region on June 7.
With unusually cool temperatures persisting in recent days, and a dearth
of
sunny weather, one cannot help but ask, what exactly is going on ?
According to the Japan Meteorological Agency, the answer is blowing in
the wind.
Wide areas of the Kanto-Koshin and Tohoku regions have been seeing
atypical summer weather in recent days owing to a double-whammy of cold,
wet winds from a high-pressure front over the Okhotsk Sea and a rainy season
front, the agency said.
At the same time, a high-pressure front that brings warmer temperatures
in summers has been unable to reach the main island of Honshu from the
Pacific,
likely owing to the influence of El Nino.
As a result, Japan may be in for a relatively cooler summer than usual.
In central Tokyo, the daily high stayed below 25 degrees Celsius for six
consecutive days until July 10. It was the first time for the capital
to see such cooler temperatures for the time period since 1993.
Meanwhile, the capital has seen just 5.2 hours of sunshine this month,
just above 10 percent of the average.
Kumagaya, Saitama Prefecture, saw only 2.1 hours of sunshine,
while Utsunomiya recorded only 4.7 hours.
The mild weather is expected to continue until around July 20.
The agency expects that the Pacific high-pressure front will increase
in strength and bring a rise in temperatures from around that time.
The "tsuyu" rainy season in Okinawa Prefecture has already ended
this year and is expected to end in other regions in late July.
This year's spell of cooler weather is in stark contrast to the record-breaking
heat in July last year, though the agency expects temperatures to be closer
to an average year from later this month through September.
"We don't anticipate as many extremely hot days this year, and it
might be
a cooler summer overall," an agency official said.
Japan invokes export controls
against S. Korea
Friday, July 5, 2019
The Japanese government on Thursday invoked
a measure to tighten export controls against
South Korea on three items, including hydrogen
fluoride needed to produce semiconductors and
other devices.
Under the measure, exporters are required
to apply to the government for each shipment,
and screening of the applications will take
about 90 days.
The move could affect Japanese companies,
such as those in the electronics industry, that
do business with South Korean manufacturers.
The three items are hydrogen fluoride, which
is used to clean semiconductors; fluorinated
polyimide, used in smartphone displays; and
resist, a photosensitizing agent for semiconductor
substrates.
Japan holds a 70 percent to nearly 100 percent
share of these materials in the global market.
South Korean companies, including Samsung
Group and LG Group, procure almost all their
supply of the materials from Japan.
Since 2004, the Economy, Trade and Industry
Ministry had waived applications for individual
licenses to export these items to South Korea
for three years, in principle.
However,.this preferential treatment has been halted.
From now on, permission must be sought
for every export contract by filing an application
with the ministry.
South Korea is said to have reserves of only
about one month's supply of the three items.
Production of semiconductors and organic
electroluminescent panels could be delayed
in South Korea starting in August, and
that could deal a blow to the South Korean
economy, for which semiconductors are
a key industry.
The measure could also affect television production
by Panasonic Corp. and Sony Corp., which use
organic EL panels produced in South Korea.
Output of Apple Inc.'s iPhone could like-wise be
affected.
Furthermore, the government has started
the process of revising a Cabinet order
to remove South Korea from the list of so-called
"white countries" that can receive preferential
treatment that simplifies export procedures,
and to require individual export applications
for items other than these three materials.
The government intends to revise it by the end
of August after seeking comment from the public.


Amsterdam mayor plans
to overhaul red-light district
Wednesday, July 6, 2019
Amsterdam mayor plans to overhaul red-light district
Femke Halsema, Amsterdam's first female mayor
launched plans to overhaul the city's red-light district
and its window displays, in a bid to protect sex workers
from gawking tourists.
In what would be the most radical revamp of the sex
trade there since the Dutch legalized prostitution
nearly two decades ago, Femke Halsema suggested
stopping the practice of sex workers standing
on display in window-fronted rooms entirely.
She said changes were needed because of social
shifts including the rise of human trafficking and
an increase in the number of tourists visiting
the district and using their phones to take
and post pictures of the women.
"We're forced by circumstances because
Amsterdam changes," Halsema said in an interview
before the launch.
"I think a lot of the women who work there
feel humiliated, laughed at - and that's one of
the reasons we are thinking about changing."
Four main scenarios are under consideration:
①ending street window displays,
②stepping up the licensing of window workers,
③reducing the number of city-center brothels, and
④closing them down altogether and moving them
elsewhere.
The scenarios, drawn up in a report titled
"The Future of Window Prostitution in Amsterdam,"
also included a broader proposal for an "erotic city
zone" that would have a clear entrance gate,
similar to a system used in Hamburg, she added.
The options will be presented to residents and
businesses at town hall meetings this month
before one is chosen and put to a vote in the city
council later this year.
Past efforts to control the red-light district
have been opposed by sex workers and businesses
involved in the trade.
The mayor said there were no plans to outlaw
prostitution outright.
Official upper house campaign
begins today
Thursday, July 4, 2019
The official House of Councillors election campaign
starts today, with the future of the social security
system, including the pension system, likely
becoming the largest point of contention.
The ruling Liberal Democratic Party and its
coalition partner Komeito are expected to aim
for a majority of seats by calling for "the stability
of politics."
Four opposition parties, including the Constitutional
Democratic Party of Japan, will unite against
the ruling camp by fielding joint candidates
in all of the 32 constituencies nationwide
with one seat up for grabs.
A Yomiuri Shimbun survey showed as of Tuesday
that about 370 people would run in the election.
There were 389 candidates in the 2016 upper
house election. The ratio of female candidates
in past upper house elections reached its
highest at 27.6 percent in 2001, but a higher
percentage is expected for the upcoming election.
The election campaign will last for 17 days
before the voting and vote counting on July 21.
Based on the revised Public Offices Election Law
that added six seats to the upper house total,
the number of seats to be contested in this
election has increased by three to 124 seats
(74 single-seat constituencies and 50 proportional
representation).
Work until you're 75 - or even 81 -
under Government review of state pension age
June 30, 2019
Millions of people could be forced to work
until they are 75, the Government has hinted
as details of a review into the state pension age
were published.
Ministers have announced a radical review of
the pensions regime amid concerns that he
current system is not "affordable in the long-term".
The Office for Budget Responsibility, the financial
watchdog, has forecast that the pension age
will have to rise to 69 by the late 2040s
before increasing again to 70 by the early 2060s.
And Royal London, the pensions provider,
has just published research suggesting that
today's workers will need to retire as late as 81
to enjoy the same standard of living enjoyed
by their parents.
The findings raise the prospect of some people
having to "work until they drop" to sustain their
current lifestyles.
The government said the review, required
under existing legislation, would consider
changes in life expectancy as well as wider
changes in society and "make sure that
the state pension is sustainable and affordable
for future generations."
It said it would also consider whether
"the current system of a universal state pension age"
rising in line with life expectancy was "optimal
in the long run".
This suggests the review will look at whether
the retirement age should rise even if life
expectancy slows.
"Any such suggestion will be of real concern
to workers across the country that the Government
is in the early stages of abandoning its commitment
to fairness for those who have worked hard all their lives.”
The year-long review will consider ending "universal"
state pension age, instead basing it on different
groups of workers and even regional living standards.
It raises the possibility that manual workers in some
areas might be allowed to claim their pension
earlier than those who have spent their working lives
in offices in other places.
It came as a minister suggested pensions tax relief
should be used to help lower paid workers rather than
those who are better off, adding to expectations
that George Osborne is preparing to mount a raid
on the pensions of middle-class savers.

Negative effects of trade conflict
will be major focus
The Yomiuri, June 25, 2019
The first summit of the Group of 20 major economies
to be held in Japan is just a few days away.
Leaders of major advanced and emerging economies
will gather in Osaka on June 28-29, aiming to take
concerted action on trade, the environment and
other issues.
On the sidelines of the summit, a number of bilateral
meetings are planned that will have an impact
on the global economy and world order.
These articles explore the expected direction of
discussions concerning major topics on the agenda.
The escalating trade conflict between the United States
and China is casting a shadow over the world economy.
One of the major focal points of the upcoming G20
summit in Osaka is whether leaders will be able
to mutually recognize the conflict's negative effects
and agree to cooperate to shore up the economy.
With U.S. President Donald Trump and Chinese
President Xi Jinping scheduled to hold talks on
the sidelines of the summit, attention is now
focused on whether their talks will help ease
tensions between the two countries.


An earthquake measuring upper 6 on the Japanese seismic intensity scale
of 7 struck off
Yamagata Prefecture on Tuesday night.
A total of 26 people had been reported injured in Yamagata, Niigata, Miyagi
and Ishikawa
prefectures as of 9:45 a.m. Wednesday, according to the Fire and Disaster
Management Agency.
Damage to houses was also confirmed as the effects of the quake came to light.
Heavy rain started in some areas. A notice to prepare for evacuation due to the risk of
landslides was issued Wednesday to 216 households in Murakami, Niigata
Prefecture,
where the quake registered an upper 6 and a heavy rain warning was issued.
The National Police Agency had not received any reports of fatalities.
The disaster agency said
a person in Tsubame, Niigata Prefecture, was seriously injured, while 10 people in Tsuruoka,
Yamagata Prefecture, suffered minor injuries.
According to a tally by The Yomiuri Shimbun, more than 7,200 residents
evacuated
in the three prefectures of Yamagata, Niigata and Ishikawa, where a tsunami warning
was announced. The Japan Meteorological Agency said the depth of the quake's epicenter off
Yamagata Prefecture was about 14 kilometers, and its magnitude was 6.7.Speech
Trump to hold trade talks with Xi at G20
WASHINGTON, Wednesday, June 19 , 2019
U.S. President Donald Trump said he'll hold trade
talks with Chinese President Xi Jinping next week
at a summit of nations in Japan.
And U.S. and Chinese negotiators will resume talks
before the leaders meet.
Investors have been on edge since the world's two
biggest economies last month broke off talks aimed
at settling a dispute over U.S. allegations that China
steals technologies and forces foreign companies
to hand over trade secrets.
The United States says the predatory tactics are
part of Beijing's aggressive drive to supplant
American technological dominance.
Trump has already imposed 25 percent tariffs
on $250 billion in Chinese imports.
And he's preparing to target the $300 billion
in Chinese imports that he hasn't already hit
with tariffs, extending them to everything China
ships to the United States.
China has retaliated with tariffs on U.S. goods.
"Had a very good telephone conversation
with President Xi of China," Trump tweeted.
"We will be having an extended meeting next week
at the G20 in Japan. Our respective teams will begin
talks prior to our meeting."
The White House said in a statement that
the two leaders discussed leveling the playing
field for U.S. farmers, workers and businesses
through a "fair and reciprocal" economic relationship.
The White House said that includes addressing
barriers to trade with China and achieving
meaningful reforms that can be verified and enforced.
Departing the White House for a campaign rally
in Orlando, Fla., Trump said:
"Our people are starting to deal as of to morrow.
The teams, they're starting to deal.
" The administration provided no details about talks
between the negotiating teams.
One of Trump's top economic advisers, Larry Kudlow,
would not speculate on what will happen
at the Trump-Xi meeting on the sidelines of the G20.
"Talk is better than no talk," said Kudlow, director of
the National Economic Council.


Hong Kong witnessed the largest protest since
it's 1997 handover to China on Sunday as huge
crowds massed against plans to allow extraditions
to the main land, a proposal that has plunged
the city's pro-Beijing leaders into a crisis.
Organizers said more than a million people
marched in blazing summer heat through
the cramped streets of the financial hub's main
island in a noisy, colorful demonstration calling
on the government to scrap its planned extradition law.
The demonstration was the biggest the international
finance hub has experienced since it was returned
to China by Britain -beaten only by a 1.5 million-
strong rally during colonial rule in 1989 supporting
the Tiananmen protesters.
Hong Kong's pro-Beijing leaders are pushing
a bill through the legislature that would allow
extraditions to any jurisdiction with which it
does not already have a treaty -including
mainland China.
But the proposals have sparked an outcry and
birthed an opposition that unites a wide cross-section
of the city.
"The government cannot ignore these numbers,"
protester Peter Chan, 21, told AFP.
"If they really choose not to response to our demands
we will not rule out more action."
For more than six hours on Sunday dense crowds
snaked their way through the city chanting
"Scrap the evil law!" and "Oppose China extradition!,"
the lines of white-dressed demonstrators
stretching for kilometers.
There are 1,030,000 people at today's march,"
an organizer told crowds outside the city's
legislature at the march's end, prompting
a cacophony of cheers and applause as new
arrivals continued to join.
Police, who historically give much lower figures than
organizers, put the peak crowd size at 240,000 -
still their second-highest estimate for attendance
at a protest since handover.
The city's population is around 7.3 million and
the organizer figure for Sunday's protest outstripped
2003, when an estimated half a million demonstrators
forced the government to shelve a deeply
unpopular national security law.
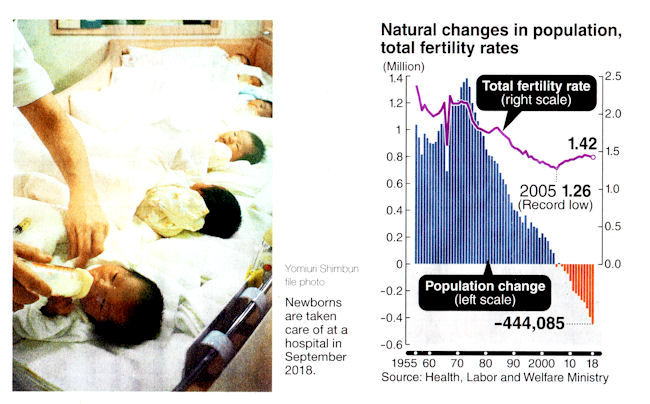
Natural population decline
exceeded 400,000 in 2018
Sunday, June 9, 2019
The government on Friday released a rough calculation
of vital statistics for 2018, revealing that the number
of deaths minus births, or natural population decline,
totaled 444,085, exceeding 400,000 for the first time.
The total fertility rate, which indicates the estimated
number of children a woman will have in her lifetime,
stood at 1.42, declining for the third consecutive year.
As the population is expected to continue to decline,
support for child-rearing and improvement of labor
productivity will continue to be key issues.
The number of deaths was 1,362,482, up 22,085
from the previous year, recording the highest
since the end of World War II.
The number of births stood at 918,397, down
27,668 from the previous year, marking the lowest
since statistics began in 1899.
As a result, the natural decrease was the largest
ever, increasing by 49,753 from the previous year.
The population saw a natural increase of 458,208
in 1989, the first year of the Heisei era.
But it took a downward turn in 2005, when it
declined by 21,266.
The number increased by 8,224 in 2006, but
has been constantly decreasing since 2007.
G-20 Officials
to Discuss Population Aging
Tokyo, June 3, 2019 (Jiji Press)
At their meeting this weekend, finance ministers and
central bank chiefs of the Group of 20 advanced
and emerging economies are expected to share
a sense of crisis over the impacts on the economy
and financial systems from the aging of population,
which is advancing globally, informed sources said Monday.
Japan, with the world's fastest-aging population,
aims to lead discussions on the effects on state
finances and economic growth of the problem
as the chair of the two-day meeting in the south-
western Japan city of Fukuoka from Saturday,
the sources said.
Likely agenda items will also include financial services
for elderly people. The meeting is designed to lay
the groundwork for the G-20 summit in the western
Japan city of Osaka on June 28-29.
According to an estimate by the United Nations,
the number of people aged 60 or over is projected
to reach two billion in 2050 worldwide, accounting
for 20 pct of the global population.
This has given rise to concerns about a fall in working
populations and low economic growth.
"How to maintain social security services in an aging
society with a declining birthrate is the most urgent
issue," Bank of Japan Governor Haruhiko Kuroda has said.

Why Mount Everest so crowded?
BBC, May 24, 2019
If you imagine the summit of Mount Everest,
you might picture a quiet, snowy peak far from civilisation.
But a striking photo, taken by mountaineer Nirmal Purja,
shows how the reality can be a lot more crowded.
The picture gives a glimpse into the tough conditions
facing climbers at the highest peak in the world.
Is it normal to see such long queues near the summit?
Yes - according to guides, this happens quite often
during the climbing season.
"It's normally that crowded," says Mingma Sherpa,
chairman of Seven Summits Treks, adding that
climbers sometimes queue between 20 minutes,
and 1.5 hours, in order to reach the summit.
It often depends on how long the window for suitable
climbing weather is - because mountaineers need
to avoid fierce jet streams that would hinder them.
"If there's one week [of safe weather], then
the summit isn't crowded. But sometimes,
when there's only a window of two or three days,
it gets very crowded" as all the climbers try to reach
the summit at the same time, Mingma Sherpa tells the BBC.

Declining birth rates
May 27, 2019
For decades, demographers and economists have known that rising incomes
are closely related to declining birth rates.
The reasons are several, and generally worth celebrating.
In developing countries, the rates of female education and access
to contraceptives tend to be lower.
As incomes rise, women acquire options outside of the home and
greater control of their reproductive rights. Affluent singles put off
marriage and children during prime childbearing years.
Meanwhile, in large cities, growing families face the burden of
high housing prices and shrinking home sizes. Those who have families
must find the resources to pay for childcare or sacrifice career options.
Longer-term, they face the burden of expensiveschool and university fees.
As a result, fertility declines are common
across the developed world. Nowhere has the
shift been more dramatic than in Asia.
For example, Japan's fertility rate has declined from
a post-World War II high of 4.4 to around 1.4 now.
In Singapore, the fertility rate dipped be-
low replacement level in the mid-1970s and
now stands at around 1.2.
And in South Korea,
the fertility rate has fallen from over 6 in 1960
to 0.95 (likely the world's lowest) during the
third quarter of 2018.
In each of these countries, the declines
have for decades been a matter of intense
concern and policy experimentation.
Governments in Asia first focused on pro-
marriage and pro-natalist policies designed
to counter what were thought to be the
factors inhibiting couples from marrying
and having children.
Singapore's efforts on this front date back
to 1984, when the government established a
matchmaking agency for university
graduates. Over the years, official initiatives
have expanded to include paying cash bo-
nuses to families for having children (the
more kids, the more lucrative), generous
maternity and paternity leave policies,
childcare subsidies and other benefits.
In Japan, pro-natalist policies were first intro-
duced in 1991 and many go further than
Singapore's; for instance, men can qualify
for a year of partially paid paternity leave.
And in South Korea, spending on pro-
natalist policies adds up to around half of
the defense budget.
There is much to recommend such
policies. Among other benefits, they boost
gender equity at home and in the workplace.
For those reasons alone, they're
worth considering in the United States, too.
But the fact is that they've failed to meaningfully increase
fertility rates.
As a result, Asia's wealthiest economies are
increasingly turning to immigration as a short
and long-term means of addressing worker shortages.
It's not easy. Singapore's government suffered a backlash
after the foreign-born population on the tiny island rose too quickly.
Japan and South Korea are much more homogenous, and home
to large constituencies that would prefer they remain that way.
But thelong-term demographic outlook for both
countries is so dire that leaders are finally getting traction
on reforms to allow more out-siders.
In Japan, where the population is shrinking,
immigration increased for the sixth straight
year in 2018; foreigners now account for 1.76
percent of the population.
Notably, more than half of these immigrants are in their 20s
and 30s -prime working years.
Meanwhile, in historically xenophobic South Korea,
the government has spent handsomely on programs
to promote multiculturalism since the mid-2000s,
while opening the doors to immigrants.
The results have been impressive: Between 2006 and 2016,
the foreign-born population grew from 536,000 to more than 2 million,
and by 2020 it's estimated that one-third of all children born
in South Korea will have a foreign-born parent.
Cool-headed talks needed to avoid
chaos from U.S.-China trade friction
Friday, May 17, 2019
The intensifying confrontation between the United States
and China significantly amplifies uncertainties in the future
of the global economy.
Their trade conflict should be solved by thoroughly
and repeatedly having cool-headed talks.
U.S. President Donald Trump announced that, effective
Friday, punitive tariffs on $200 billion (about Y22 trillion)
worth of Chinese goods will be raised from 10 percent
to 25 percent.
Trump seemingly aims to extract further concessions
from China at ministerial-level trade negotiations
between the two countries that will start Thursday.
Optimistic views had recently been spreading in markets
that an agreement would be reached soon during
bilateral negotiations.
The Trump's sudden announcement of tariff hikes
reversed these views.
Investor sentiment has quickly deteriorated and has
caused a spontaneous, worldwide decline in stock values.
In the United States, the Dow Jones Industrial Average
temporarily dropped nearly 650 points on Tuesday.
On Wednesday in Tokyo, the Nikkei Stock Average
also dove 321 points.
The United States and China account for about 40 percent
of global gross domestic product (GDP).
If their confrontation intensifies with the tariff increase,
that would bring an even more serious level of damage
to the markets and economic activities.
A situation should be avoided in which the trade dispute
gets bogged down.
In the bilateral ministerial-level trade negotiations,
both countries need to make concessions to avoid
the raising of punitive tariffs.
Nissan forecasts profit will drop
to 11-year low
Wednesday, May 15, 2019
Nissan Motor Co. forecasts a 28 percent plunge
in its annual operating profit, setting it up
for the weakest earnings in 11 years and
underscoring its struggle to turn the page
after the ouster of former Chairman Carlos Ghosn.
The lackluster outlook from Japan's No.2 automaker
- hit by Ghosn's arrest last year and roubles
at its North American business - is likely to add
to the pressure on CEO Hiroto Saikawa as he tries
to overhaul corporate governance and put Nissan
on a more equal footing with alliance partner Renault.
Nissan's weakening profit has raised concerns
at Renault, which holds a 43 percent stake
in the Japanese firm and has pushed for closer ties.
But some Nissan executives have opposed a full
merger and what they see as an unequal partnership
that gives smaller Renault more sway over Nissan.
"Today we have hit rock bottom," Saikawa told
a news conference at the company's headquarters
in Yokohama on Tuesday.
"We would like to recover to our original performance
level in two to three years," he added.
Nissan expects operating profit of Y230 billion
($2 billion) for the year to March 2020, missing
the Y457.7 billion average of 23 analyst estimates
compiled by Refinitiv.
U.S.-China trade war would be
bad for whole world
Wednesday, May 8, 2019
Warren Buffett said Monday that a trade war
between the United States and China would be
"bad for the whole world."
Buffett spoke after U.S. President Donald Trump
tweeted Sunday that he will raise tariffs on $200
billion of Chinese imports to 25 percent from 10
percent beginning Friday.
Trump said he would soon slap a 25 percent tariff
on $325 billion of Chinese goods that have not been
taxed.
Major stock markets fell worldwide Monday
in response to the tweet, which preceded scheduled
trade talks this week, and was a "rational" response,
Buffett said on CNBC.
Buffett's conglomerate Berkshire Hathaway Inc.
owns or invests in many companies that do business
in China, including Apple Inc., in which it has a more
than $50 billion stake, and Chinese electric car maker
BYD Co.
"If we actually have a trade war it will be bad
for the whole world," Buffett said.
A full-scale trade war is unlikely, he said, but
"would be bad for everything Berkshire owns."
Berkshire Vice Chairman Charlie Munger told CNBC
that Trump is not "totally crazy" for wanting higher
tariffs on some goods, but a trade war would be
"massively stupid."
Despite the concerns, Buffett said it would be
"nonsense" for investors to sell stocks based
on negative headlines, adding that the United States
and China will be the world's superpowers for the next
100 years and will always have tensions.
He said the battle would not affect how Berkshire
operates. Berkshire owns more than 90 companies
including utilities, makers of industrial parts and
chemicals, Geico insurance and Dairy Queen, and
ended March with $191.8 billion of equity investments.
78% support
symbolic emperor system
May 4, 2019
A recent Yomiuri Shimbun nationwide survey showed
78 percent of the respondents said Japan should
maintain the system in which an emperor is recognized
as the symbol of the state as it is now, while 7 percent
said such a system should be abolished.
5percent said the status of the emperor as the head
of state should be clarified and his authority should be
strengthened, while 8 percent said they have no interest
in the matter.
The recent survey was conducted between March 12
and April 18 by mail before the change of the era
from Heisei to Reiwa.
Yomiuri surveys on the Constitution started in December
1989, in the first year of the Heisei era.
(They were conducted by interview back then.)
At that time, 73 percent said that Japan should maintain
the system.
Differences between past and present survey methods
make direct comparisons difficult, but support
for the symbolic emperor system can be said to have
remained at a high level.
Reiwa era starts
May 1, 2019
It's a new era in Japan, the Reiwa era.
Emperor Naruhito has begun his reign
as his father abdicated.
It's a historic development,
the first Imperial succession from a living Emperor
in more than 200 years.
Emperor Naruhito is now the 126th Emperor of Japan.
Later on Wednesday morning, he'll take part
in ceremonies at the Imperial Palace
to mark his enthronement.
At 10:30 a.m., the new Emperor will take part
in a ceremony to show he's inherited
the Imperial Regalia, a sacred sword and jewel,
as proof of his enthronement.
About 30 minutes later, he'll meet with
representatives of the people.
They include the Prime Minister, the heads of
both chambers of the Diet, the Chief Justice of
the Supreme Court, and local government leaders.
Emperor to abdicate today
April 30, 2019
The Emperor abdicates Tuesday under the special
measures law on the Imperial House Law that
enables the abdication of the current emperor.
It will be the first abdication in about 200 years
since that of Emperor Kokaku during the Edo period,
and the first under the constitutional system of
government.
The Emperor is to attend the Taiirei-Seiden-no-gi
abdication ceremony at the Imperial Palace,
which will take place at 5 p.m. on Tuesday.
The Emperor and the Empress carried out their last
official duty before his abdication away from
the Imperial Palace on Friday, attending
the Ceremony of Awarding the Midori Prize hosted
by the Cabinet Office.
According to the Imperial Household Agency,
the Emperor was to stay at the Imperial Palace
through Monday as no official duties have been planned.
On Tuesday morning, the Emperor will attend
a ceremony to report his abdication at
the Kashikodokoro Imperial ancestor's shrine,
where the sun goddess Amaterasu Omikami,
the Imperial family's ancestral deity, is enshrined.
Kashikodokoro is one of three Imperial shrines.
Home vacancy rate
hits record high
April 28, 2019
The proportion of vacant homes in total housing units
in Japan stood at 13.6 percent as of Oct. 1 last year,
hitting a record high, a government survey showed Friday.
The estimated number of vacant homes increased
to a record 8.46 million, the internal affairs ministry
survey also said.
The number of vacant homes not marketed for rental
or sale came to 3.47 million units, or 41.4 percent of
the total vacant homes.
The share expanded consistently from 2003.
Japan's total housing units rose by 1.79 million units
from the previous survey in 2013.
Vacant homes increased by 260,000 units.
But the vacancy rate saw only a marginal rise of 0.1 point.
The result reflected a decrease in vacant homes
in urban areas, including Tokyo and Aichi Prefecture,
a ministry official said.
By prefecture, Yamanashi saw the highest vacancy
rate of 21.3 percent, followed by Wakayama at 20.3
percent and Nagano at 19.5 percent.
Meanwhile, Saitama and Okinawa had the lowest rate,
at 10.2 percent, followed by Tokyo at 10.6 percent.
A reason for the high rates in Yamanashi and Nagano,
which have popular resorts, is that there are many
secondary houses, such as holiday homes.
Excluding this factor, Wakayama had the highest
home vacancy rate of 18.8 pcircent, followed
by Tokushima at 18.6 percent and Kagoshima
at 18.4 percent.
The Results apparently stemmed from aging and
decreasing local populations.
Both vacant home numbers and vacancy rates
grew in Iwate, Miyagi and Fukushima, which all
suffered heavy damage from the March 2011
earthquake and tsunami.
The growth reflected increasing demand for home
rebuilding thanks to progress in postdisaster
reconstruction, the official said.
The government takes the survey every five years
from 1948. The latest one, the 15th of its kind,
covered about 3.7 million homes and made
estimates for the nationwide situation.
The surveyed homes were limited to those where
people can live, not including those abandoned or
in danger of collapse.

Putin said:
Kim probably not to shut
his nuclear program
April 26, 2019
Russian President Vladimir Putin said after holding
his first face-to-face talks with North Korean leader
Kim Jong Un on Thursday that U.S. security
guarantees would probably not be enough
to persuade Kim to shut his nuclear program.
Putin and Kim held a day of talks on an island off
the Russian Pacific city of Vladivostok two months
after Kim's summit with U.S. President Donald Trump
ended in disagreement, cooling hopes of a break-
through in the decades-old nuclear row.
The talks between Putin and Kim did not appear
to have yielded any major breakthrough.
Putin, keen to use the summit to burnish Russia's
diplomatic credentials as a global player, said
he believed any U.S. guarantees might need to be
supported by the other nations involved in previous
six-way talks on the nuclear issue.
That would mean including Russia, China, Japan and
South Korea as well as the United States and
North Korea, a long-standing format that has been
sidelined by unilateral U.S. efforts to broker a deal.
“ The North Koreans only need guarantees about
their security. That's it. All of us together need
to think about this,” Putin told reporters after talks
with Kim.

the Islamic State still now
has strong influenc
April 25, 2019
Though the Islamic State lost in territory, but
it did not lose in ideological influence.
"I don't think it's too soon to say that defeat of
the physical caliphate in Iraq and Syria was never
going to be the end of the ISIS challenge," said
Nicholas Rasmussen, a former senior director
for counterterrorism on the National Security
Council who also ran the National Counterterrorism
Center in the Obama and Trump administrations.
"That is why many terrorism experts urged care and
restraint on the administration in making claims
about defeat of ISIS.
The ideology underpinning the caliphate has reach
far beyond Iraq and Syria."
Its influence has also spread to places where
it hasn't traditionally held sway.
Last week, through its Amaq News Agency, the group
asserted responsibility for an attack in Congo for
the first time.
For decades, parts of eastern Congo have been
submerged in conflict, and a number of armed groups
seeking to undermine the government have taken
advantage of the chaos, launching attacks on civilian
and military targets.
In a statement, John Manley, a spokesman for U.S.
Africa Command, said it now considers one of those
groups, the Allied Democratic Forces (ADF), to have
"meaningful ties to the Islamic State.
" The group claimed allegiance to the Islamic State
in 2017, John Manley said.
A V.S. official who works on the region said that
the ties should be taken seriously, and that relationships
between senior ADF leaders and foreign extremist
groups go back decades.

U.S.Force in S.Korea stages
THAAD Missile Defense Drill
April 24, 2019
The USFK have staged several THAAD exercises this year
but in Pyeongtaek rather than Seongju,
North Gyeongsang Province where they are stationed.
It is not clear whether the USFK had moved a THAAD
launcher from Seongju or used a different launcher
for practice.
A senior South Korean military officer said.
"Basically, THAAD is a mobile anti-missile defense
system. The USFK's latest exercise in Pyeongtaek
seems intended to send a message that the U.S.
will defend its key facilities here."
The U.S. has been ratcheting up pressure
on North Korea by rattling its saber since
a U.S.-North Korea summit collapsed in Hanoi.
The stationing of the THAAD system here in early 2017
got South Korea into trouble with China, which
fears that its powerful X-band radar could be used
to spy on Chinese military maneuvers and launched
a yearlong unofficial boycott of Korean goods and
services in response.
Zero-energy plan
for all housing units
The Yomiuri Shimbun, April 23, 2019
The government plans to achieve zero-energy status
for all housing units in Japan by early in the latter half
of this century through promotion of solar power and
other energy-saving features, it has been learned.
The goal will be part of a long-term strategy
the government is required to map out in accordance
with the Paris Agreement, an international framework
to combat global warming.
The government plans to announce the goal at a meeting
of the Central Environment Council on Tuesday.
Such housing units are called Net Zero Energy Houses,
also known as ZEH. The government aims for almost
all newly built housing units to be ZEHs by 2030,
and will promote them through government subsidies
and other means.
In the strategy, the government will also call for
achieving zero-energy balanced status for existing
houses units through renovations and other measures.
The strategy will be compiled based on proposals made
by a government panel of experts on April 2.
The panel proposed that the nation must become
a decarbonized society as early as possible
in the second half of this century.
In addition to realizing ZEHs, the government also
will promote the use of car sharing as a norm
in people's everyday lives.
In the energy field, the government will advance
development of small nuclear reactors on the condition
that safety will be prioritized over all else.
In the industry sector, cutting-edge technology will be
developed to remove oxygen from iron are using hydrogen
instead of coal, as greenhouse gas emissions
by the steel industry account for about 15 percent
of overall domestic emissions.
The government will solicit views from the public
by late May, and plans to formally adopt the long-term
strategy by the time of the Group of 20 major economies
summit talks to be held in Osaka in June.
Among the Group of Seven major countries, only Japan
and Italy have not yet compiled their strategies.
Elderly living alone estimate
to top 30% in 2040
Tokyo, Japan, April 21, 2019
The share of single-person households in elderly households,
or those headed by people aged 65 or over, will be above
30 percent in all of Japan's 47 prefectures in 2040,
an estimate by a welfare ministry think tank showed Friday.
Citing the reason for the estimate, an official of
the National Institute of Population and Social Security
Research said, "Elderly people who have never married
will increase as the number of people opting not to marry
started to rise in the 1980s."
The institute estimated prefecture-by-prefecture numbers
of households, including elderly households, during
the period until 2040, based on the results of the 2015
national census.
The proportion of elderly households in the total households
will likely be above 30 percent in all prefectures in 2030
and above 40 percent in 45 prefectures, excluding
Tokyo and Aichi, in 2040, according to the institute's survey.
The proportion in 2040 is projected to be the highest
in Akita, at 57.1 percent.
Nine other prefectures are also estimated to record
figures exceeding 50 percent.
The proportion of single-person households in elderly
households already topped 30 percent in 24 prefectures
in 2015.
In 2040, the proportion is projected to exceed 40 percent
in 15 prefectures, with Tokyo topping the list, at 45.8
percent, followed by 45.4 percent in Osaka, 44.8
percent in Kochi, 44.6 percent in Kagoshima and
43.1 percent in Hokkaido.
10-day holiday makes
41% of people unhappy
Jiji Press, April 16, 2019
A recent Jiji Press poll found that some 40 percent of
people in Japan are not happy about an unprecedented
10-day Golden Week holiday from late April to early May
to commemorate the Imperial succession.
The survey showed that a total of 36.5 percent of
respondents are happy about the long holiday,
with 9.3 percent choosing the answer "very happy"
and 27.2 percent "happy to some extent."
Meanwhile, a total of 41.0 percent were found
to be unhappy, with 14.8 percent saying that they are
"not happy at all" and 26.2 percent "not very happy."
Respondents not interested in the 10-day holiday
made up 21.1 percent.
With the Emperor scheduled to abdicate on April 30
and Crown Prince Naruhito to ascend the throne
the following day, the government has made
the entire period from April 27 until May 6
a special holiday period.
The proportion of people in their 30s who are not
happy about the 10 days of stood at 46.9 percent.
The percentage stood at 45.9 percent for those in
their 40s.
Asked about reasons why they are not happy
with multiple answers allowed, the largest group,
or 28.0 percent, said that the holidays do not matter
to them because they are not working, followed
by 19.3 percent who cannot take days oil during
the holiday period, 10.8 percent whose burdens
such as household work will increase and 9.6 percent
who worry that the holidays may disrupt their
business operations.
The survey also found that during the holiday period,
32.4 percent expect to take 10 straight days off,
while 15.0 percent cannot or will not take any day off.
The proportion of respondents who can or will take
only a day off stood at 4.3 percent.
People who will take two to three days of accounted
for 18.5 percent.
The percentage of men who cannot or will not take
any day of stood at 9.4 percent, while that of women
came to 21.0 percent.
Regarding ways to spend the holidays, the largest
group, or 64.3 percent, will stay at home to have
a relaxing time.
Respondents who plan to do household work,
go shopping or watch movies in nearby areas
accounted for the majority.
The proportion of respondents who will travel
in or outside Japan stood at 16.2 percent.
The survey was conducted March 8-11 through
interviews with 2,000 people aged 18 and over
nationwide.
Valid answers were given by 61.2 percent.
Britain, don't waste extra time
given to untangle Brexit mess
April 12, 2019
The worst-case scenario of Britain leaving the European
Union without a deal has been averted for the time being,
but it is not obvious how this mess can be untangled.
This time around, Britain must swiftly show a responsible
solution.
The EU has held an extraordinary summit meeting and
decided to extend the deadline by which Britain will
leave the bloc until the end of October at the latest.
Britain had been scheduled to depart the bloc Friday.
If Britain's Parliament ratifies the draft Brexit deal reached
by the British government and the EU, an earlier Brexit
will be allowed.
British Prime Minister Theresa May's ruling Conservative
Party has been unable to develop a consensus on the deal,
and it cannot be said for certain when or if it will be ratified.
May has held talks with the Labour Party, the largest
opposition party, in an attempt to find a breakthrough,
and she sought an extension until the end of June.
However, the EU decided the prospects of making
progress so quickly were slim.
European Council President Donald Tusk quite rightly
demanded of Britain, "Please don't waste this time."
If Britain repeatedly kicks the Brexit can down the road,
its international prestige will be harmed and the number
of companies leaving the country likely will accelerate.
Britain's House of Commons had been unable to garner
majority sup port for any of the concrete Brexit proposals
offered, and has ceased to function properly.
Will the government and the La bour Party both make
compromises and find a plan that will be passed by
Parliament?
Will steps such as a change of prime minister break this
deadlock?
It is vital that Britain quickly shows a way forward.
Japan's population
down for 8th straight year
TOKYO, April 14, 2019
Japan's estimated population including foreigners
totaled 126,443,000 as of Oct. 1 last year,
down 263,000, or 0.21 percent, from a year before,
falling for the eighth straight year,
the Internal Affairs and Communications Ministry
said Friday.
The decline was the largest ever,
both in number and percentage.
The number of people aged 15 to 64, or the working
age population, fell by 512,000 to 75,451,000,
accounting for 59.7 percent of the overall population,
the lowest level since comparable data became available
in 1950, highlighting labor shortages in Japan.
The share of the working age population has been
on the decline since hitting a peak of 69.8 percent
in 1992. The percentage in 1950 was also 59.7 percent.
The population of Japanese people came to
124,218,000, down 430,000, or 0.35 percent.
The number of foreigners increased by 167,000,
or 8.1 percent, to 2,225,000, rising for the sixth
straight year. The share of foreigners of the
overall population has been on the rise.
People aged 65 or older were 28.1 percent of
the overall population, hitting a record high.
Those aged 70 or older stood at 20.7 percent,
the first reading above 20 percent.
Of the 47 prefectures, seven saw their populations
increase, with a rise of 0.72 percent in Tokyo,
0.31 percent in Okinawa and 0.28 percent
in Saitama.
The Tokyo metropolitan area, which also includes
Kanagawa, Chiba and Saitama prefectures,
enjoyed continued population growth.
The population decreased in 40 prefectures,
falling 1.47 percent in Akita, 1.22 percent
in Aomori and 1.12 percent in Iwate.
The pace of decline was faster in 2018 than
a year before in Wakayama and 30 other
prefectures.
Okinawa was the only prefecture with a natural
population increase, with the number of births
exceeding that of deaths.
S. Korea court orders
abortion ban be lifted
The S.Korea News, April 12, 2019
South Korea's Constitutional Court on Thursday
ordered the country's decades-old abortion ban
to be lifted in a landmark ruling over a law that
campaigners say puts women at risk.
South Korea remains one of the few industrialized
nations that criminalizes abortion, except
for instances of rape, incest and when the mother's
health is at risk.
But the nine-member bench ruled by seven to two
that the 1953 statute aimed at protecting lives and
traditional values "goes against the Constitution"
and ordered the law to be revised by the end of
next year.
"The abortion ban limits women's rights
to pursue their own destinies, and violates their
rights to health by limiting their access to safe
and timely procedures," the court said in a statement.
"Embryos completely depend on the mother's
body for their survival and development,
so it cannot be concluded that they are separate,
independent living beings entitled to rights to life."
Bursting into tears of joy and celebrating,
hundreds of Women - including teenagers and
females with disabilities - cheered wildly in front of
the Constitutional Court in central Seoul, where
the official ruling was announced.

Japanese banknotes get a makeover
Wednesday April 10, 2019
On Tuesday, Japanese finance minister Taro Aso announced that Japanese banknotes
will be getting a redesign. The new notes are expected to enter circulation
around 2024,
the first change since 2004.
Three historical figures have been chosen for the new designs, selected
based
on several guidelines.
They include that the figures must be widely known in Japan, that their
achievements
be such that the Japanese people take pride in them, and that precise pictures of them
exist so as to prevent forgeries.
There are a couple exceptions to the makeover. The 2000 yen note will remain
unchanged,
as there are not many in circulation, while the 500 yen coin will be redesigned
around 2021.
The global economy
may need stimulus measures
April 11, 2019
The global economy is slowing more than expected
and a sharp downturn could require world leaders
to coordinate stimulus measures, the International
Monetary Fund said Tuesday as it cut its forecast
for world economic growth this year.
The global lender's semi-annual World Economic
Report pointed to the U.S.-China trade war and
a potentially disorderly British exit from the European
Union as key risks and warned that chances of
further cuts to the outlook were high.
Some major economies, including China and Germany,
might need to take short-term actions, the IMF said.
"This is a delicate moment for the global economy,"
IMF chief economist Gita Gopinath said in a news
conference to discuss the report.
Governments may need to open their pocketbooks
at the same time "across economies" if the slowdown
becomes more serious, Gopinath said, adding
that loose monetary policy might also be needed.
The comments provided an eerie warning to the
global officials gathering in Washington this week
for the spring meetings of the IMF and World
Bank.
The world engaged in coordinated fiscal stimulus
to counter the 2008 financial crisis.
In its third downgrade since October, the IMF said
the global economy will likely grow 3.3% this year,
the slowest expansion since 2016.
The forecast cut 0.2 percentage points from the
IMF's outlook in January.
The projected growth rate for next year was
unchanged at 3.6%.
More than two-thirds of the expected slowdown
in 2019 stems from troubles in rich nations,
including members of the EU.
"In this context, avoiding policy missteps that
could harm economic activity should be the main
priority," the IMF said in its report.
One potential misstep lies in Britain's indecision
over how to leave the EU.
Despite looming dead lines, London has not decided
how it will try to shield its economy during the exit
process.
The IMF's new forecast assumes an orderly Brexit,
but the Fund said a chaotic process could shave
more than 0.2 percentage points from global growth
in 2019.
It said the Bank of England should be "cautious"
on its interest rate policy, an apparent tip to wait
before hiking borrowing costs.
The EU's economic growth is already slowing
substantially, though the IMF said it still expects
the slowdown in Europe and some emerging
market economies will give way to a re
acceleration in growth during the second half of
2019.
The outlook for Germany, one of the main drivers
of European growth, suffered from weaker
demand for its exports, softer consumer spending
and new emissions standards that have depressed
car sales.

A young Korean's lonely feeling
April 1, 2019
Dark Moon, 33, moved to Seoul 12 years ago.
His family has been living in Busan.
He has been living alone since then.
It took him a while to accustom himself
He has become used to the solo life.
However, he feels there is one thing that disturbs him.
"Eating alone, sleeping alone and watching the TV
alone is easier year by year, but as years go by,
a feeling of loneliness swoops over me more
frequently at unexpected moments," he said.
"For example, when I return home after a long day
at work and turn on the light at my empty studio
apartment, I suddenly feel as if I am alone in the
world."
The lonely feeling eventually deepened to depression.
The office worker now sees a therapist once a week
to improve his mental health.
"There could be many reasons for my depression,
but I think I would manage it well if I had someone
to talk to and casually share my daily life with every
day," Dark Moon said.
According to Statistics Korea,
there were about 5.6 million one-person households
in South Korea in 2017, accounting for about 29 percent
of total households.
In 2000, the percentage of one-person households
recorded 15.5 percent.
Statistics Korea expected the rate to surpass
36 percent in 2045.
Meanwhile, a Hankook Research survey of 1,000
adults in South Korea conducted in April 2018,
showed 46 percent of people who live alone
felt lonely "always" or "frequently" and
44 percent felt lonely sometimes.
All told, almost 90 percent of single-person
households are exposed to a feeling of loneliness.

Japanese government selects
"Reiwa " for the new era name
April 1, 2019
"Reiwa" is a name that will be on the lips of most Japanese today
and it will be for years when Crown Prince Naruhito becomes
the new Emperor on May 1.
The announcement was highly anticipated here because it will define
the years ahead as well as play a daily role in people's lives.
Chief Cabinet Secretary Yoshihide Suga said, "We'll work towards
getting the new era name widely accepted and deeply rooted in the lives
of the Japanese people."
The new name was taken from Manyoshu, the oldest existing anthology
of Japanese poetry.
It comes from a passage that can be translated as:
"In early spring, the air is fresh and the wind is calm.
The plum flowers are blooming like a beautiful woman applying white powder
in front of the mirror. And the fragrance of flowers is like that of robes
scented with incense."
Prime Minister Shinzo Abe says the name represents the hope that
every Japanese person will achieve their aspirations just like a plum flower
flourishing after a severe winter.
He said, "Culture is nurtured when people beautifully bring their hearts together.
Reiwa has that meaning."
Diet OK's record-high budget
for 7th consecutive year
March 27, 2019
The Diet enacted the government's fiscal 2019
initial budget on Wednesday, including a \2,028 billion
stimulus package, amid growing uncertainties
over the global economy.
In the initial budget, general-account spending
totaled \101,457.1 billion, topping the \100-trillion mark
for the first time ever while rewriting the record high
for the seventh consecutive year.
“We'll do all we can for the early implementation
of the budget,” Prime Minister Shinzo Abe told reporters.
“We're resolved to make full efforts in economic
policy management as uncertainties are growing
over the world economy.”
The swollen budget mainly reflects increasing social
security expenditures against the backdrop of
the aging population, as well as the costs of
the stimulus package.
The budget also includes defense spending totaling
a record \5,257.4 billion, including on the deployment
of Aegis Ashore land-based missile interceptor systems.
The stimulus package, designed to alleviate the impact
of a planned consumption tax hike in October, features
a rebate program for cashless payments and the issue of
discount vouchers for those on low incomes.
For the two projects, a total of \450 billion was earmarked.
The package also included \1.3 trillion for public works
projects for disaster prevention.
On top of the stimulus package, the government plans
other measures including making preschool education
free of charge.
“We'll take more-than-enough measures to guarantee
[the continuation of] the economic recovery,” Abe has
told the Diet.
Since the government drew up the fiscal 2019 budget,
however, recent economic figures have indicated that
overseas economies were in worse shape than initially
expected.
In October-December, foreign demand remained
a negative contributor to Japanese gross domestic product
for the third straight quarter.
In a monthly report released earlier this month,
the Cabinet Office downgraded its basic economic
assessment for the first time in three years.
Ahead of a series of local elections in April and
a House of Councillors poll in the summer,
the government may face growing calls from the ruling
bloc to implement further stimulus measures promptly,
observers said.
Selection of new era name
in final stage
News, 2019.3.25
Japan's government has revealed that on March 14
it officially selected multiple experts to consider
a new name for the era of the nation's next emperor.
Chief Cabinet Secretary Yoshihide Suga's
announcement on Sunday suggests that the selection
process is now in the final stage.
The government will decide the new era name
on April 1 by holding a meeting of experts.
Emperor Akihito will abdicate the throne on April 30,
and Crown Prince Naruhito will ascend the throne
the following day, May 1.
The procedure for selecting a new era name requires
the prime minister to nominate a panel of experts and
academics to come up with a shortlist of two to five
options for each panel member.
The Chief Cabinet Secretary will then examine
the shortlist and present several candidates
to a panel of representatives from various fields.
Suga will also hear the opinions of speakers and
vice-speakers of the both chambers of the Diet.
A new era name will be finalized after a Cabinet
meeting convenes to approve a government
ordinance that will alter the name.

`Year of the Boar'
unified elections under way
Japan News , March 23, 2019
Gubernatorial elections were officially announced
in 11 prefectures Thursday, kick-starting campaigning
for nationwide unified local polls.
With the population and industry becoming
increasingly concentrated in the Tokyo metropolitan
area, candidates will spend the next month or so
competing over their visions for reinvigorating regional
economies and halting population decline.
These so-called Year of the Boar elections feature
unified local polls and a House of Councillors election
in the same year - something that happens once
every 12 years.
The local elections will be a key barometer of how
the ruling and opposition parties might fare
in this summer's upper house poll.
According to The Yomiuri Shimbun's calculations,
about 980 elections will be held to choose heads
of local governments and local assembly members.
On Thursday, gubernatorial elections were officially
announced in Hokkaido, Kanagawa, Fukui, Mie,
Osaka, Nara, Tottori,Shimane, Tokushima, Fukuoka
and Oita.
A total of 30 people including eight incumbents
had filed their candidacies by the 5 p.m. deadline.
Pay careful attention to the risk of
changes in global economy
2019.3.22
Won't this situation cause the risk of an economic slow-
down? Now is the time for careful discernment.
The government has revised down its assessment of
the economy for the first time in three years in its
monthly economic report for March.
It has changed its statement assessing the overall economy
from "mildly recovering" to "mildly recovering although
weakness is seen recently in exports and some parts
of production."
This seems to have stemmed mainly from the economic
slowdown in China, which is amajor trading partner of
Japan.
Exports of electronic parts and other products are sluggish,
thus putting a brake on companies' production.
If this slowdown in exports continues, it could cool corporate
capital investment. The government states it is highly likely
that the economic expansion period marked in January is
the longest in postwar history. Yet doubt about that
view is growing stronger.
The government and the Bank of Japan should levelheadedly
analyze the economic circumstances while avoiding
prejudgment.
Uncertainty over the situation abroad, such as the trade
friction between the United States and China and issues
related to Britain's exit from the European Union, could
also weigh on the economy.
BOJ Gov. Haruhiko Kuroda has presented a view that
the Chinese and European economies will improve
in the latter half of this year.
In contrast, no optimism is warranted about the future of
the U.S. economy due to the nation's political turmoil.
It is necessary to pay the utmost attention to changes
in the world economy.
The key is how to vitalize domestic demand.
Consumer spending, which accounts for the greater part of
Japan's gross domestic product, lacks strength. Substantial
pay raises were realized in such industries as logistics and
retail, which are suffering from serious labor shortages,
but it is a cause of anxiety that moves to raise wages
are slow in the manufacturing industry in particular.
Air pollution 'killing more people
than smoking'
European Repo, 2019.3.21
Air pollution is killing more people every year than smoking,
according to research published on March 12 that called
for urgent action to stop burning fossil fuels.
Researchers in Germany and Cyprus estimated that air
pollution caused 8.8 million extra deaths in 2015-
almost double the previously estimated 4.5 million.
The World Health Organization estimates smoking kills
about 7 million people a year globally.
The researchers found that in Europe - the key focus of
the European Society of Cardiology research - air
pollution caused an estimated 790,000 deaths, between
40 percent and 80 percent of them from cardiovascular
diseases such as heart attacks and stroke.
"Since most of the particulate matter and other air pollutants
in Europe come from the burning of fossil fuels, we need
to switch to other sources for generating energy urgently,"
said coauthor Prof. Jos Lelieveld, of the Max-Plank Institute
for Chemistry in Mainz and the Cyprus Institute Nicosia.
"When we use clean, renewable energy, we are not just
fulfilling the Paris Agreement to mitigate the effects of
climate change, we could also reduce air pollution-related
death rates in Europe by up to 55 percent."
The study, published in the European Heart Journal,
focused on ozone and the smallest pollution particles,
known as PM2.5, that are particularly harmful to health
as they can penetrate into the lungs and may even be able
to cross into the blood.
The researchers said new data indicated the hazardous
health impact of PM2.5 - the main cause of respiratory
and cardiovascular disease - was much worse than
previously thought.
They urged a reduction in the upper limit for PM2.5
in the European Union, which is currently set at 25
micrograms per cubic meter, 2.5 times higher than
the WHO guideline.
"In Europe the maximum permissable value ... is much
too high," said Lelieveld and coauthor Prof.Thomas Munzel,
of the Department of Cardiology of the University Medical
Centre Mainz in Germany, in a joint statement.
Putin signed
'anti-fake news' laws
Russian repo, 2019.3.20
Russian President Vladimir Putin on Monday signed
controversial laws that allow courts to fine and briefly
jail people for showing disrespect toward authorities,
and block media for publishing "fake news."
Putin signed off on the legislation against the advice of
human rights activists, who warned the laws amounted
to censorship and would be abused to further crack down
on freedom of speech.
The law on disrespecting authorities backs punishment
for "offending state symbols" and stipulates hefty fines
and jail terms of 15 days for repeat offenders.
Another piece of legislation allows authorities to decide
what amounts to "fake news" and gives a media
watch-dog the power to demand an outlet delete
the information.
Websites that fail to comply would be blocked.
Fines could reach 1.5 million rubles (more than $22,700)
if the infraction leads to grave consequences like death
or rioting.
Build defenses against cyber-attacks
2019.3.18
If it is necessary to protect social and economic systems
from serious cyber-attacks threats.
The government must expedite arrangements to do so.
central government offices, local governments and
private firms are exposed to severe attacks in cyber-
space.
In 2017, a type of malware called ransomware spread
across the world, causing hospitals, railway companies,
banks and big businesses in Japan to suffer damage
such as temporary suspension of their operations.
Cyber-attacks on Japanese government organizations
amount to 7 million a year.
The government will shortly establish a cyber security
council consisting of government offices, local governments
and critical infrastructure operators, including power
utilities, railway firms and airport operators.
This is the first attempt to share cyber-attacks information
widely between the public and private sectors.
Confidentiality agreements will be imposed on the participants
to make it easier for firms that have suffered cyber-attacks
to reveal the methods used.
This will lead to preventing similar cyber-attacks from
expanding and causing damage.
The planned panel will also look cyber-attacks cooperation
at establishing a system in which the government or
a business detects cyber-attacks and sends alerts instantly
so that information can be shared.
It is highly significant that a diverse range of organizations
cooperate with each other and take appropriate steps.
Even as lawmakers vote to delay Brexit,
no way seen to avert chaos
World News, 2019.3.17
Britain has been unable to decide on a course over
its exit from the European Union-an issue that has
put the fate of the country at stake-as the government
and Parliament have fallen into disarray. The political
turmoil in Britain is serious.
The House of Commons passed a motion to postpone Brexit,
with the scheduled departure approaching on March 29.
Given the risk of a "no-deal exit," which could disrupt
the economies of both Britain and the EU, it can be said
there was no alternative but to delay Brexit.
The problem is that British Prime Minister Theresa May's
political clout has been on the decline.
The draft withdrawal agreement reached between the British
government and the EU was voted down by a wide margin
on March 12, following a defeat in a similar vote in January.
Theresa May was forced to give up her stance of proceeding
with Brexit as scheduled and proposed a motion to ask the
EU for a delay.
In the vote on the motion, about 60 percent of lawmakers
of the ruling Conservative Party, including some Cabinet
members, voted against the delay. But parliamentary
approval of the postponement was won with support
from Labour, the largest opposition party.
Theresa May expressed her intention to put the draft
withdrawal agreement to a vote again next week.
If the agreement is approved, Brexit will be postponed
until the end of June.
If it is rejected, Theresa May is said to ask the EU
for a longer extension.
It appears that pressure has been placed on hard Brexiteers
in the Conservative Party, who oppose the draft withdrawal
agreement, that voting it down again could lead to Brexit
being canceled.
The menace of plastic waste
The Japan News, 2019.3.12
WWF International is calling on Southeast Asia and other
regions to tackle the menace of plastic waste, with its
director general outlining a multi-pronged approach
and sustainable solutions.
The world produces and consumes more than 300 million
tonnes of plastic products every year.
Marco Lambertini said the menace of plastic waste has
spun out of control because of the unprecedented growth
in popalations and the middle class, especially in China,
India and southeast Asia.
About half of all plastic items are discarded after being
used just once. Less than 15 percent of plastic is recycled.
As a result, plastic waste has become an enormous threat
to the environment, with about 8 million pieces of micro-
plastic and other forms making their way into the oceans
annually.
This garbage is posing a serious threat to marine life and
in turn to human health due to the micro and nanoplastic
that ends up in seafood.
In addition, the economic cost of plastic waste is growing
rapidly thanks to sewage and drainage blockages, and is
causing ecological damage at tourist attractions such as
beaches.
The underlying issue is people using too much plastic and
discarding it too carelessly. It is convenient and cheap and
disposability is built in.
According to Lambertini, the best way to tackle this problem
would be to impose taxes - primarily on manufacturers so as
not to burden consumers.
He said reducing the overall consumption of plastic is one of
the three priorities, with a focus on unnecessary packaging.
Large retailers can play a key role in changing consumer
behavior, he said, as in the recent campaign at 7-Eleven
convenience stores, which managed to convince shoppers
to forego plastic bags and thus far has kept 100 million
bags out of circulation.
Hesei-Era Lesson
The Japan News ,Febuary 22, 2019 By Prof.Yoshikawa
Widening inequality
During the three-decade-long Heisei era, we have undergone
the collapse of the bubble economy, the ensuing financial
crisis, the Izanami boom, the Lehman Brothers shock,
the Great East Japan Earthquake and Prime Minister
Shinzo Abe's growth strategy known as Abenomics.
Two phenomena have occurred in tandem with these
developments:
①the decline in the birthrate and the rapid aging of
the population,
② the widening of inequality.
While reasons for inequality vary from country to country,
this phenomenon is aggravated in Japan largely due to
the aging of the population.
Inequality in society tends to worsen when there is an increase
in the number of elderly citizens whose levels of income,
assets and health vary widely from person to person.
What is noteworthy in this connection is the widening
of inequality even among the country's working generations.
At the start of the Heisei era, one in every six employees was
a non-regular worker, but non-regular employees now
account for nearly 40 percent of the overall workforce.
Society security is the system purported to ease the problem
of inequality.
At the outset of the Heisei era, social se curity benefits
amounted to about ¥47 trillicin a year, while
the comparable annual total now reaches - ¥120 trillion,
a 2.5-fold increase.
Unfortunately, social security insurance premiums cannot
fully finance such benefits -they cover about 60 percent of
the entire sum. The shortfalls are-filled by public funds.
However, tax revenues stop far short of making up
for the necessary coverage.
As a result, the shortage of fiscal resources for social
security spending emerges fully as the source of the state's
fiscal deficit.
Time
to fight climate extremes
The Japan News ,by Akihiko Tanaka, Feburary 18, 2019
China and the United States must reduce their green-
house gas emissions, which are the world's largest.
In 2015,China accounted for 28.4 percent of the world's
emissions, while the United States accounted for 15.4
percent.
Japan's figure was 3.5 percent.
The reason the Paris Agreement was praised as an epoch-
making breakthrough is that both China and the United
States signed the pact, obliging themselves to contribute
to climate change mitigation.
However, U.S. President Donald Trump has declared
that he will withdraw the United States from the Paris
Agreement.
Generally speaking, China continues to be positive about
the agreement. But dark clouds are looming over what
China will eventually do regarding the climate agreement
due to the currently worrying international circumstances.
Such concern reflects the fact that relations between
Washington and Beijing have become so tense that
it can be said that a new Cold War has begun.
January, 2019 turned out to be a month of extreme weather,
the World Meteorological Organization (WMO) said
in a recent report.
In southern Minnesota, the temperature dropped to minus
53.9 C on Jan. 30, breaking the previous U.S. low temperature
record of minus 48.9 C.
What the WMO calls "high-impact weather" events also
gripped the southern hemisphere in a totally different
way.
Australia, for instance had its warmest January on record
with Adelaide hitting 46.6 C, the hottest reading for
any Australian state capital.
The fact that many parts of the northern hemisphere
were recently enveloped by bone-chilling cold waves
may make us think that global warming is not progressing
anymore. It is.
What is really happening is an increase in extreme
weather occurrences caused by climate change.
In recent weeks, the United States saw the Midwest
suffering from extreme cold even while warmer-than-usual
temperatures continued in Alaska - and in the peripheral
zones of the Arctic as well.
Last summer, Japan, too, had extreme weather.




In L.A., more homeless people died than in N.Y.
L.A. had great cold weather, more homeless died here than in N.Y.
February 18, 2019
One homeless was found near a homeless shelter and taken to Los Angeles
County-USC Medical Center, where he later died.
Seemingly improbable factor contributed to his death.
Hypothermia, or loss of body heat, from being out in the cold.
One of the abiding myths about Los Angeles is that homeless people
come here from the East Coast or Midwest because at least they won't
freeze to death.
But despite L.A.'s typical sunshine and mild temperatures, five homeless
people died of causes that included or were complicated by hypothermia
in the county last year, surpassing San Francisco and New York City,
which each reported two deaths. Over the last three years, 13 people
have died at least partly because of the cold, the coroner's office said.
And advocates worry that this cold, rainy winter will mean more fatalities.
24 years on, lessons
from Great Hanshin Earthquake
still crucial
The Yomiuri Shimbun, Thursday, January 17,2019
The lessons from two major disasters that struck the nation
during the Heisei era must be passed to the next generation.
Thursday marked 24 years since the Great Hanshin Earthquake,
which is inscribed in the annals of Japanese disasters along
with the Great East Japan Earthquake.
The experience of losing 6,434 lives in the Hanshin quake
became the foundation for measures to deal with disasters
that remain in place today.
The initial response to the Great Hanshin Earthquake has been
widely criticized. It took about six hours after the quake hit
for the Prime Minister's Office to finally get an accurate picture
of the extent of the damage.
The Hyogo prefectural government, which was overwhelmed
as it responded to the disaster, turned down offers of assistance
from other local governments, and it took at least four hours
for the governor to request the Self-Defense Forces
be dispatched to help.
Soul-searching on these shortcomings resulted
in the establishment of the crisis management center
at the Prime Minister's Office, and mutual support between
cities, wards, towns and villages also took root.
The disaster can be said to have become an opportunity to
press the central and local governments to prepare disaster
management systems.
After the Great East Japan Earthquake, the request to
dispatch SDF personnel came six minutes after the temblor.
Accurate information is necessary so SDF personnel can
swiftly rush to a disaster-hit area and save many
lives.
Cooperation with local governments and police forces must
be deepened during ordinary times.
The disaster also made people aware of the importance
of self-help, in which people protect themselves, and
mutual assistance, in which local residents help each other
in addition to public assistance provided by central and
local governments.
 Visit of the General Public to the Palace
Visit of the General Public to the Palace
for the New Year Greeting
Thursday, Jan.3, 2019
Members of the public visit the Imperial Palace to offer
New Year congratulations for the last time in the Heisei
era on Wednesday. About 154,800 visitors, the most
for a New Year Greeting in the Heisei era, gathered
from early morning, prompting the Emperor and
Empress to appear at the balcony of Chowa-Den Hall
a total of seven times, two more times than scheduled.

 Indonesia tsunami:
Indonesia tsunami:
Fears of new wave as Anak Krakatau volcano seethes
Monday, December 24, 2018
At least 373 people have been killed and 1459 injured after a tsunami
hit coastal towns on Indonesia's Sunda Strait, government officials say.
There was no warning of the giant waves which struck at night,
destroying hundreds of buildings, sweeping away cars and uprooting
trees.
It is thought undersea landslides from the Anak Krakatau volcano
caused them.
President Joko Widodo has expressed his sorrow for the victims
and urged people to be patient.
Rescue efforts are being hampered by blocked roads
but heavy lifting equipment is being transported to badly hit
areas to help search for victims.
The Sunda Strait, between the islands of Java and Sumatra,
connects the Java Sea to the Indian Ocean.
What do we know so far?
The disaster management agency has warned people to stay away
from the coastline due to fears of another tsunami.
Saturday's tsunami struck at about 21:30 local time (14:30 GMT),
during a local holiday.
It hit several popular tourist destinations including the Tanjung
Lesung beach resort in the west of Java island.
◾As it happened: Indonesia tsunami
Footage shared on social media showed a large wave crashing
into a tent in the resort, in which popular Indonesian rock band
Seventeen was performing. Members of the band were seen
being swept away as the wave destroyed the stage.
In a tearful Instagram video, singer Riefian Fajarsyah said
the band's bassist and road manager had died, and three other
band members and his own wife were missing.
◾Survivors' stories
Red Cross official Kathy Mueller told the BBC: "There is debris
littering the ground, crushed cars, crushed motorcycles,
we're seeing buildings that are collapsed."
It appears that the main road into Pandeglang has been badly
damaged, making it difficult for rescuers to reach the area,
she added.
Eyewitness Asep Perangkat said cars and containers had been
dragged about 10 metres (32 feet).
"Buildings on the edge of [Carita] beach were destroyed,
trees and electricity poles fell to the ground," he told AFP
news agency.
Officials say more than 160 people were killed in Pandeglang
- a popular tourist district on Java known for its beaches and
national park.
Meanwhile, 48 were reported dead in South Lampung on Sumatra,
and deaths were also reported in Serang district and Tanggamus
on Sumatra. Officials fear the death toll could rise further.

 Aerosol spray may be cause of disaster in Sapporo
Monday, December 17, 2018
Aerosol spray may be cause of disaster in Sapporo
Monday, December 17, 2018
SAPPORO Police believe an explosion at a Sapporo building which
left 42 people injured on Sunday was caused by gas leaking
from more than 100 deodorizer spray cans meant for disposal
at a real estate agency.
The wooden building, which housed the agency, a Japanese-style
pub and a clinic, collapsed in the blast, local authorities said.
The explosion occurred at around 8:30 p.m. Sunday,Dec.16,2018,
in Toyohira Ward, resulting in a fire that was extinguished at 2:10 a.m.
An employee of the real estate agency told police about the disposal
of the spray cans, and it is suspected that the gas from the cans
ignited when a water heater was switched on.
Among the 42 victims, a male employee of the real estate agency
in his 30s was seriously injured. That office was the most severely
damaged. The injured included 19 men and 23 women, aged
from 1 to their 60s, according to the police.
Numerous customers who were dining on the pub’s second floor
said they smelled gas at the time of the explosion.
Windows of condominiums and restaurants near the site,
which is located close to a subway station in the Hokkaido capital,
were shattered, and wood debris was scattered dozens of meters
away.
Many people who felt the blast said they thought it was an earthquake.
The Fire and Disaster Management Agency said it has dispatched
seven officials to the site to look into the cause.
A local fire department said the three tenants in the building
were poorly prepared for fires, as authorities found
no equipment for evacuations or alarms there during an on-site
inspection in October. The department first learned of the poor
preparation two years ago and asked the tenants to take
measures on six occasions — but the requests were ignored.
A gas safety center official who visited the blast site said five tanks
of propane gas weighing 50 kg each were installed outside of the pub,
while two 20-kg tanks were located outside the real estate office.
Gas pipes in the buildings were confirmed to be damaged after
the explosion.
"The ceiling fell, then the entire second floor collapsed and
we were all stuck. We managed to escape after everyone kicked
through the wall,” a person who was in the pub at the time and
later treated at a hospital said, according to a relative.
The ground floor of the pub had a kitchen and counter seats
while the second floor had private dining rooms, according to
a neighbor who frequented the establishment.
“Suddenly I heard the boom, my body was lifted and blown
to the floor,” said a 28-year-old man who was dining
with a friend on the second level of the pub. “After the explosion,
power went out and fire instantly spread and came near
so I jumped out of an open window to the street.”
“If the second floor hadn't collapsed, everyone might have been
stuck there and burned to death,” said a 49-year-old man who
was dining with his family at the pub. Fire spread within five minutes
of the blast, throwing customers into a state of panic with screams
and cries, he recalled.
The man said he had a brush with death in the face of approaching
flames, but the second level collapsed shortly afterward and
he fell to the street.
“Orange flames leaped up and the pub was blown away,”
a neighborhood resident said, adding that upon rushing outside
he saw that the building was completely destroyed.
“Immediately after the sound of the explosion, windows were broken
and bits of glass hit me. I got cuts on my face and hands,”
a 68-year-old woman who was dining at a nearby restaurant said
after receiving treatment at a hospital.
“All the food on the table was blown away.”
Kunihiko Yanazume, 76, head of a hospital about 70 meters away
from the explosion site, said pieces of shattered glass were scattered
all over and that the automatic door at the entrance was broken.
“We may not be able to reopen the hospital this year,” he said.
More than 50 people temporarily evacuated to a nearby public facility
offered by the municipal government.
“Everything is a mess inside the house and I don't know where
to start,” said a 34-year-old woman who was staying at the evacuation
center with her two children.
 9 killed in train collision in Turkey
Thursday, December 13, 2018
9 killed in train collision in Turkey
Thursday, December 13, 2018
Nine people were killed and nearly 50 injured in Turkey
when a high-speed train collided with a locomotive and
crashed into a station platform and overpass in an Ankara
suburb early Thursday, officials said.
Rescuers worked to free people trapped under the mangled
wreckage at Marsandiz train station, eight kilometers
from central Ankara.
It was not clear at what speed the train and locomotive were
traveling when the collision occurred.
There was light snow on the tracks.
The train had been heading from Ankara to the central Turkish
province of Konya and was not due to stop at Marsandiz.
Ankara Gov. Vasip Sahin said the locomotive, which lay
battered 20 meters further ahead, carried out track inspections.
Three train drivers were among the nine killed in the crash,
Transport Minister Cahit Turhan told reporters on the scene.
There were 206 passengers on the high-speed train,
according to state-owned Anadolu news agency, which also
reported that the Ankara state prosecutor's office
had launched an investigation.
U.S. automakers call for more open Japan market
December 12, 2018
Detroit automakers and labor unions on Monday insisted that
any U.S. trade deal with Japan contain strong provisions
to combat currency manipulation and pry open Japan's largely
closed auto market before lowering any U.S. autos tariffs.
At a hearing on U.S. negotiating objectives for trade talks
expected to start early next year, the United Auto Workers
union called on the Trump administration to demand imposing
strict quotas on Japanese vehicles and parts, with any increases
based on the growth of U.S. automotive exports to Japan.
Desiree Hoffman, UAW international representative, said that
despite zero tariffs on vehicle imports, Japan's auto market is
largely closed as a result of other barriers such as Japan-specific
regulatory, safety and emissions standards and currency
manipulation to push down the value of the yen.
"These barriers have created an uneven playing field so much
so that for every car that the U.S. exported to Japan in 2017,
Japan sent 100 back," Hoffman said.
"Any loosening of the 2.5 percent automotive or 25 percent
light truck tariff would further direct Japan's overcapacity
to our shores, exacerbating the problem."
Currency provisions stronger than those negotiated in the new
U.S.-Canada-Mexico free-trade deal are needed in a U.S.-Japan
trade agreement, said Matt Blunt, president of the American
Automotive Policy Council, which represents Ford Motor Co.,
General Motors Co. and Fiat Chrysler.
Given Japan's history of currency market intervention, he said,
"more enforceability" was needed with clearer penalties for
violating currency provisions. He said in the past, AAPC has
advocated that trading partners with high current account
surpluses and high foreign currency reserve levels be cut off
from free-trade agreement benefits if they are found to be
intervening in currency markets.
"We recommend the administration avoid making any
concessions that would further open the U.S. market tc
Japanese imports unless and until then is evidence that
Japan is truly committed to opening its auto market to U.S
vehicles," Blunt said.
AFL-CIO trade policy specialist Celeste Drake said a U.S.-Japan
trade deal should have a sunset clause that allows termination
if it fails to achieve a 50 percent reduction in the U.S.
nonmilitary industrial goods trade deficit with Japan.
The total U.S. goods trade deficit with Japan was about
$69 billion in 2017, with autos trade accounting for about
75 percent of the gap.
Objectives for trade talks
Testimony from the hearing will feed into the U.S. Trade
Representative's formulation of negotiating objectives
for the trade talks, which U.S. President Donald Trump and
Japanese Prime Minister Shinzo Abe agreed to launch in
late September.
Japan has sought to keep discussions on currency separate
from trade agreements. The Bank of Japan has intervened
on occasion to maintain currency stability in the face of
safe-haven inflows.
A representative of foreign-brand automakers in the United
States said currency issues are better dealt with by multilateral
groups such as the Group of 20 or Group of 7 major economies.
"We don't want to limit our policy options to promote economic
growth here," said John Bozzella, president of the Association
of Global Automakers adding that such provisions could restrict
U.S. monetary policy.
Bozzella, whose organization represents major Japanese and
European automakers, said an immediate elimination of U.S.
automotive tariffs would help make U.S. auto production
more competitive by increasing access to technology and
components.
Oil nations to slash output from Jan.
World News:Monday, December 10, 2018
Oil prices spiked sharply higher Friday as major oil producers,
including the OPEC cartel, agreed to cut global oil production
by 1.2 million barrels a day to reduce oversupply.
Following two days of meetings, the Organization of the Petroleum
Exporting Countries, which includes the likes of Saudi Arabia and
Iraq, said they would cut 800,000 barrels per day for six months
from January, though some countries such as Iran, which is facing
wide-ranging sanctions from the United States, have been given
an exemption.
The balance will come from Russia and other non-OPEC countries.
The United States, one of the world's biggest producers, is not part
of the deal.
"This is a major step forward," said United Arab Emirates Energy
Minister Suhail Mohamed al-Mazrouei, who chairs the regular
meetings in Vienna in his capacity as president of the OPEC Conference.
Oil producers have been under pressure to reduce production following
a sharp fall in oil prices over the past couple of months.
The price of oil has fallen about 25 percent recently because major
producers,including the United States, are pumping oil at high rates.
The reduction has certainly met with the response hoped for
by ministers as it was at the upper end of most predictions.
Following the announcement, Brent crude, the international standard,
was up $2.79 a barrel, or 4.7 percent, at $62.85.
Benchmark New York crude was $2.11, or 4.1 percent,
higher at $53.60 a barrel.
Ann-Louise Hittle, a vice president at oil industry expert
Wood Mackenzie, said the production cut "would tighten"
the oil market by the third quarter next year and help lift
Brent prices back above $70 per barrel.
"For most nations, self-interest ultimately prevails," she said.
"Saudi Arabia has a long-term goal of managing the oil market
to avoid the sharp falls and spikes whichthurt demand and the
ability of the industry to develop supply.
On top of this, Saudi Arabia also needs higher oil revenues
to fund domestic Saudi spending."
Russian Energy Minister Alexander Novak called the negotiations
with the OPEC nations "fairly challenging" but said the decision
"should help the market reach a balanced state."
"I think this is a strong signal to anybody who has doubted
that our cooperation is continuing and we can react to any
challenge the market throws at us," he said in Russian through
a translator.
OPEC's reliance on nonmembers like Russia highlights the cartel's
waning influence in oil markets, which it had dominated for decades.
The OPEC-Russia alliance was made necessary in 2016 to compete
with the United States' vastly increased production of oil in recent
years.
By some estimates, the Unied States this year became the world's
top crude producer.
The cut is unlikely to be greeted warmly by U.S. President Donald
Trump, who has been pressuring the cartel publicly to maintain
production.
On Wednesday, he tweeted: "Hopefully OPEC will be keeping oil
flows as is, not restricted. The World does not want to see, or
need, higher oil prices!"
One stumbling block to an agreement had been Iran, Saudi Arabia's
regional rival and fellow OPEC member, which had been arguing
for an exemption to any cuts because its crude exports are
already being pinched by U.S. sanctions.
Diet approves trade deal with EU
Sunday, December 9, 2018
The Diet approved an economic partnership agreement
with the European Union, which will create a free trade bloc
accounting for 30 percent of world output on Saturday.
The House of Councillors passed the pact by a majority vote
at a plenary session, with support mainly from the ruling
coalition of the Liberal Democratic Party and Komeito.
The House of Representatives gave its approval late last month.
Japan is expected to finish the necessary procedures, including
amending governmental and ministerial ordinances, by the end
of this year.
With the European Parliament slated to ratify the trade agreement
by year-end, the EPA is seen coming into effect on Feb. 1, 2019.
This will follow the effectuation of the Trans-Pacific Partnership
free trade agreement among 11 nations, including Japan and
Australia, slated for Dec. 30 this year.
The Japanese government hopes to demonstrate the superiority
of multilateral free trade regimes over bilateral ones before
it kicks off bilateral trade talks with the United States next year,
officials said.
Under the EPA, tariffs will be removed for over 90 percent of trade
items between Japan and Europe.
Tariffs will decline for a wide range of agricultural, forestry and
fishery products, including European cheese,wine and pork,
allowing Japanese consumers to enjoy such goods at lower prices.
Japanese manufacturers will see greater opportunities to expand
their exports to the EU, because tariffs will be removed on
Japanese industrial products, including automobiles and car parts.
In Diet deliberations, opposition parties expressed concerns about
negative effects on the dairy sector from an influx of European
products, including cheese.
The government and the ruling bloc promised to work on measures
to bolster the competitiveness of the domestic agricultural sector,
including support for dairy farmers.

 France fears more riots;
museums, Eiffel Tower to close today
Repo:Saturday, Decemger 8, 2018
France fears more riots;
museums, Eiffel Tower to close today
Repo:Saturday, Decemger 8, 2018
Authorities across France braced Thursday for the possibility of
more riots and violence at anti-government protests this weekend,
holding emergency meetings and deploying tens of thousands of
police and security forces.
Museums, theaters and shops in Paris announced they would close
Saturday as a precaution, including the city's famed Eiffel Tower.
Police unions and city authorities met to strategize on how to handle
the protests on Saturday, which are being held even though French
President Emmanuel Macron surrendered Wednesday night and
cancelled a fuel tax hike that had unleashed weeks of unrest.
On the other side of France's volatile social debate, disparate
groups of protesters did the same thing, sharing their weekend
plans on social networks and chat groups.
French Prime Minister Edouard Philippe told senators Thursday
that the government will deploy "exceptional " security measures
for the protests in Paris and elsewhere.
Speaking on TF1 television, Philippe said 89,000 police officers
will be deployed on Saturday across France,up from 65,000
last weekend.
In Paris alone, 8,000 police officers will be mobilized.
They will be equipped with a dozen armored vehicles,
first in a French urban area since 2005.
Some "yellow vest" protesters, French union officials and
prominent politicians across the political spectrum called for
calm Thursday after the worst rioting in Paris in decades
last weekend.
Macron agreed to abandon the fuel tax hike, part of his plans
to combat global warming, but protesters' demands have
now expanded to other issues hurting French workers,
retirees and students.
And in a move questioned by both critics and supporters,
the president himself has disappeared from public view.
The prime minister reiterated the government's plan to scrap
a fuel tax rise planned by the previous government because
of the "extreme tensions" France is facing.
"No tax deserves to put civil peace in danger," Philippe said.
G20 finance ministers warn
of downside risks
Saturday, December 1, 2018
Finance ministers of the Group of 20 wrapped up their discussions
on global economic issues in Buenos Aires on Thursday.
They shared the view that the world's economy is stable
but the downside risks are increasing due to factors,
including trade friction.
Japan stressed the importance of free trade.
It said the multilateral trade system, based on free and fair rules,
must be maintained to guide economic growth.
After the meeting, Japanese Finance Minister Taro Aso spoke
to reporters about the expected talks between the leaders of
the US and China.
Aso said, "We don't expect the current situation will change
significantly unless the two sides hold substantial discussions."
Aso said Chinese policies are not always fair. He said
he thinks that unless Beijing changes its stance
the US will maintain its sanctions against China.
U.S. climate report warns of disasters
Sunday, November 11, 2018
As California's catastrophic wildfires recede and people
rebuild after two hurricanes, a massive new federal report
warns that these types of disasters are worsening in the United
States because of global warming.
The White House report quietly issued Friday also frequently
contradicts U.S. President Donald Trump.
The National Climate Assessment was written long before
the deadly fires in California this month and before
Hurricanes Florence and Michael raked the East Coast and
Florida.
It says warming-charged extremes “have already become
more frequent, intense, widespread or of long duration.”
The report notes the last few years have smashed U.S. records
for damaging weather, costing nearly $400 billion since 2015.
The recent Northern California wildfires can be attributed
to climate change, but there was less of a connection to those
in Southern California, said coauthor William Hohenstein
of the U.S. Department of Agriculture.
“A warm, dry climate has increased the areas burned
over the last 20 years,” he said at a press conference Friday.
The report is mandated by law every few years and is based
on more than 1,000 previous research studies.
It details how global warming from the burning of coal,
oil and gas is hurting each region of the United States
and how it impacts different sectors of the economy,
including energy and agriculture.
“Climate change is transforming where and how we live
and presents growing challenges to human health and
quality of life, the economy, and the natural systems
that support us,” the report says.
That includes worsening air pollution causing heart and
lung problems, more diseases from insects, the potential
for a jump in deaths during heat waves, and nastier allergies.
“Annual losses in some economic sectors are projected
to reach hundreds of billions of dollars by the end of the century —
more than the current gross domestic product (GDP) of
many U.S. states,” the report says. It'll be especially costly
on the nation's coasts because of rising seas and severe
storm surges, which will lower property values. And in some
areas, such as parts of Alaska and Louisiana, coastal flooding
will likely force people to relocate.
“We are seeing the things we said would be happening,
happen now in real life,” said another coauthor Katharine
Hayhoe of Texas Tech University.
“As a climate scientist it is almost surreal.”
And Donald Wuebbles, a coauthor and University of Illinois
climate scientist, said, “We're going to continue to see
severe weather events get stronger and more intense.”
What makes the report different from others is that it
focuses on the United States, then goes more local and granular.
“All climate change is local,” said Pennsylvania State
University climate scientist Richard Alley, who wasn't part
of the report but praised it.
While scientists talk of average global temperatures,
people feel extremes more, he said.
“We live in our drought, our floods and our heat waves.
That means we have to focus on us,” he said.
The Lower 48 states have warmed 1 C since 1900
with 0.66 C in the last few decades, according to the report.
By the end of the century, the United States will be
1.6 to 6.6 C hotter depending on how much greenhouse gases
are released into the atmosphere, the report warns.Speech



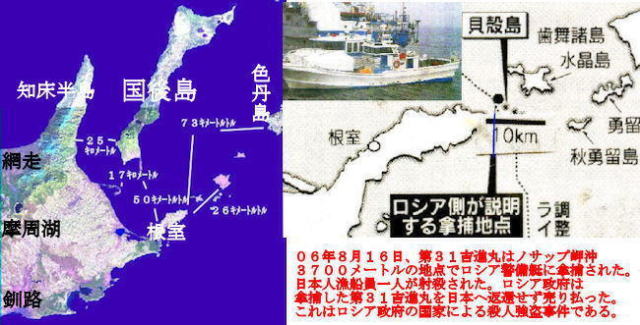
 Japan-Russia negotiations
Japan-Russia negotiations
on disputed islands
Friday November 16, 2018
On Wednesday, the leaders of Japan and Russia reached an agreement
that could mark a breakthrough in the decades-long dispute between
the countries.
Prime Minister Shinzo Abe said that he and Russian President Vladimir Putin
agreed in Singapore to accelerate negotiations on a peace treaty based
on a 1956 joint declaration.
How do experts see the agreement?
NHK World correspondent Shin Watanabe interviewed a former negotiator
of Japan and Russia, as well as a Japanese expert.
The joint declaration states that 2 of the 4 disputed islands would be
handed
over to Japan on the conclusion of a bilateral peace treaty.
The two countries never signed a peace treaty at the end of World War Two.
The stumbling block on a peace treaty has been the territorial issue concerning
4 Russian-controlled islands.
Japan claims them. The government maintains they are an inherent part of
Japan’s territory. It says they were illegally occupied after the war.
In September, Putin proposed signing a peace treaty without any preconditions
by the end of the year. Abe later said Japan couldn't do that until the territorial
dispute is resolved.
Kyoto Sangyo University Professor Kazuhiko Togo is a former high-ranked
Japanese diplomat who has negotiated with Russia. He says, "I think
this is
a historical turning point. For the first time, the 2 governments have agreed
on the fundamental structure of a possible agreement. If we do not start
now,
we will run the risk of losing time and no conclusion of any kind will
be reached.
And that would be detrimental."
Former Russian Ambassador to Japan Alexander Panov says, "Russia solved
many territorial problems. Its last one is with Japan. Putin recognizes
this
declaration is a treaty because this declaration was ratified by the parliaments
of both countries so this is absolutely a legal document which no one can ignore.
There will be some opposition, not only in Russia, but in Japan as well.
The obligation of my country to pass over these islands to Japan was agreed
upon during the Soviet era in 1956. So we're just fulfilling this obligation.
So, I think it's not so difficult to reach an agreement."
An expert of Japan-Russia relations, Hosei University Professor Nobuo Shimotomai
says, "Favorable political conditions in both Japan and Russia were probably
conducive to the agreement.
In Japan, Prime Minister Abe has extended his tenure by being re-elected
as leader of the main governing Liberal Democratic Party. President Putin
is
likely to have taken into consideration the fact that Abe is a conservative
politician and urged Abe to decide to accelerate negotiations for a peace
treaty
based on the 1956 joint declaration -- and Abe agreed.
Abe's planned visit to Russia in January also suggests that progress
in the negotiations is foreseeable to a certain extent. The agreement of
the schedule is significant in that regard.
Some difficult points in negotiations from here on will likely include
issues
such as determining the border between Japan and Russia, and deciding
which side will assume sovereignty over the 2 islands after they are returned.
It's also unclear what kind of joint economic activities will be carried out
on the 4 islands which Japan calls the Northern Territories, or
how the islands of Kunashiri and Etorofu, not mentioned in the joint declaration,
will be handled. These issues are still open to discussion."
Abe said President Putin will visit Japan for the G20 summit next year,
and before that, he will travel to Russia, possibly early next year, to
further
discuss the issue with the Russian president.
Solving this territorial dispute is one of the top priorities for the Japanese
government. But it will be a sensitive negotiation and officials from both sides
are handling it delicately to avoid a public backlash from their citizens.
Poll shows split on immigration bill
Tuesday, November 13, 2018
NHK's latest opinion poll suggests that Japanese voters are divided
over the idea of more foreign workers coming into the country.
NHK conducted its monthly opinion poll over the weekend.
More than 1,200 responded. The telephone survey asked how people feel
about a potential major shift in the country's immigration law.
During the current Diet session, lawmakers will be discussing a bill
aimed at relaxing the country's rules over foreign workers coming
into Japan. The country currently accepts highly skilled professionals
from abroad, like researchers and lawyers.
The legislation is aimed at allowing blue-collar workers into the country.
It would create two new visa categories.
One would allow foreigners engaged in simple labor to stay in Japan
for up to 5 years, but would not allow them to bring family members.
The second would allow more skilled workers to bring their families,
and extend their five year stay indefinitely.
If the bill passes, officials estimate an additional 40,000 foreign workers
would be accepted into the country in fiscal 2019.
Respondents were asked what they think about expanding the number of
foreign workers into the country. And the results suggest voters are split
over the idea.27 percent of respondents say they approved.
30 percent said they disapproved. While 36 percent said they were undecided.
Toshiba plans to fire 7,000 employees
Saturday, November 10, 2018
Toshiba Corp will cut nearly 7,000 jobs after a $1.3 billion accounting scandal,
in an overhaul that will streamline the sprawling conglomerate into a company
focused on chips and nuclear energy.
Toshiba also would sell its television manufacturing plant in Indonesia, and
that eventual job cuts spanning the entire PC-to-nuclear company could be
over 10,000 including previously announced cuts and those seeking voluntary
early retirement.
Due to restructuring costs, which include the sale of its Indonesian TV plant,
Toshiba expected a net loss of around 550 billion yen in this fiscal year ending
in March 2019.
“By implementing this plan, we would like to regain the trust of all
stakeholders including shareholders and transform ourselves into a robust
business,”Toshiba said in a statement.
Toshiba confirmed in August,2018, that it overstated profits going back to fiscal
2008/09 by 155 billion yen. It also reported a 37.8 billion yen net loss for the
last financial year to reflect more costs and conservative estimates on operations,
including the South Texas Project, a U.S. power plant project.
An independent accounting probe said in July that the company suffered
from dysfunction in governance and a culture of discouraging employees
from questioning their superiors.
Toshiba's stock has fallen about 40 percent since news of its accounting
problems began to emerge in early April,2018. The scandal and subsequent
earnings restatements highlighted weaknesses in a range of Toshiba's businesses.
Analysts have said restructuring was long overdue. The company launched
the world's first mass-market laptop in 1985 but has seen its consumer
electronics business dwindle amid price competition with Asian rivals.
The change in fortune highlights the decline of the 140-year-old conglomerate,
which remains highly influential in the Japanese business community.
Over the years, its former executives often played key policy advisory roles
in government.
 Monkeys strike for reduced quantity of the bait
Friday, November 9, 2018
Monkeys strike for reduced quantity of the bait
Friday, November 9, 2018
Even a monkey does not appear in the music hall in Mt. Takasaki Monkey
Park of Oita-shi.
It is considered to influence the power of the group which increased
dissatisfaction, and has been staying in the heart of a mountain,
and made a rally
for " strike" for the garden that has been reducing
the quantity of the bait, year by year.
This facilities originally fed the wild Japanese monkey which damaged
the farm products.
There are B & C groups.
B group is approximately 640, and C group approximately590,
inhabited in the mountain, and it was common that first,
B group appeared morning in the music hall, then C group came afternoon.
It was this spring that an accident became remarkable.
The garden has been reducing the bait per one,
little by little for approximately 30 years,
to reduce the numbers of the monkey,from 2,000 to 800.
Natually,
"Dissatisfaction to bait blew up" .
US Congress divided as Democrats win House
Thursday, November 8, 2018
Trump's Republican Party has retained control of the Senate in midterm
elections, but Democrats have retaken the House of Representatives.
The divided Congress is expected to impede Trump's efforts to implement
his agenda, including the construction of a border wall and repealing
his predecessor's health care plan.
Thirty-five of the 100 seats in the Senate and all 435 seats in the House of
Representatives were up for grabs.
Vote counting is still underway but ABC television predicts Republicans
will control 51 seats in the Senate and Democrats will have 45,
with 4 seats remaining.
ABC says Democrats are on course to add 32 to 36 seats in the House.
The Democrats focused on winning over voters by criticizing Trump
for creating social divisions over his immigration policies.
They also campaigned hard on healthcare, a major concern for voters.
Trump appears unfazed by the outcome, tweeting, "Tremendous success
tonight. Thank you to all!"
The Democrats are expected to use their control of the House to unleash
a fierce attack on Trump over alleged Russian meddling in the 2016
presidential election.
Trump said the Republican Party "defied history" in expanding its majority
in the Senate.
He spoke to reporters at the White House on Wednesday, one day after
the midterm elections.
He said Republicans achieved the result despite huge funding
for the Democrats and "very hostile media coverage."
He stressed his contribution, saying he attended 30 rallies during 60 days
to support candidates and 9 out of 11 won seats in the Senate.
The Democratic Party won a majority in the House of Representatives,
leading to a divided Congress.
Trump said Republicans and Democrats should work together.
He noted that he hopes to cooperate with the Democrats on economic growth,
infrastructure, and trade.
 Halloween misdeeds in Shibuya
Tuesday, October 30, 2018
Halloween misdeeds in Shibuya
Tuesday, October 30, 2018
Tokyo police arrested 5 people over the weekend for alleged assaults and
other misdeeds in the run-up to Halloween on Wednesday.
Police are calling on people gathering to celebrate the day to behave lawfully.
Many costumed revelers have been congregating since Saturday at the famous
scramble crossing at Shibuya Station.
Most are young adults, including some foreign tourists.
Some were drinking alcohol and causing disturbances in the road.
Some Halloween revelers overturned a small truck early Sunday.
The driver got out of the vehicle safely.
Frequent scuffles have broken out in heavily crowded areas around the crossing.
Three people have been arrested on suspicion of assault.
Two others were collared for groping and similar misconduct.
Shoppers appear to be avoiding the popular commercial district because of
the rowdy revelers.
The manager of a drugstore complained that sales are bad despite the number
of people in the area, and said store hours may be shortened on Halloween.
Extra police officers will patrol the area through Halloween on Wednesday.
Two major retail stores
to suspend liquor sales on Halloween
Thursday,October 25, 2018
Don Quijote and Lawson say their outlets in Tokyo's Shibuya district
will not sell alcoholic drinks in glass bottles on Halloween.
Major Japanese discount chain Don Quijote and convenience store operator
Lawson decided the move in response to a request from Shibuya Ward.
The local administration asked for cooperation from shops near Shibuya
Station to prevent drunken partygoers from bothering people.
In recent years, the scramble crossing in front of Shibuya Station and
the nearby streets have become a gathering point for those celebrating
Halloween on the night of October 31st.
Some residents have complained about partying and noise late
into the night and the huge amount of garbage left by the revelers.
A Don Quijote outlet near Shibuya Station will stop selling alcoholic
beverages in glass bottles on Wednesday next week through 6:00 AM
on Thursday.
Lawson will do the same at its 6 shops near the station starting at 6:00 PM
on Wednesday until 6:00 AM on Thursday.
Nobel laureate Tasuku Honjo hopes
Japan invests more in science
Tuesday, October 2, 2018
Nobel Prize-winning scientist Tasuku Honjo voiced hope on Tuesday
that Japan would invest more in science, a day after he was chosen
for this year's award in physiology or medicine along with American
James Allison for their studies on cancer therapy.
“I was able to prove that it is not rare for fundamental research
to lead to applications,” Honjo, 76, said at a news conference held
at Kyoto University, where he is currently a professor.
“Science is an investment for the future.”
News that Honjo became the 26th Japanese Nobel Prize winner was met
with a shower of praise from cancer patient groups and the Japanese
government on Monday.
“Cancer patients are being saved by (the new cancer medicine) Opdivo,
which originated from a study carried out by the Japanese researcher.
We are delighted that it was positively evaluated,” said Shinsuke Amano,
head of the Japan Federation of Cancer Patient Groups.
When new medicine is developed abroad, it usually takes time to gain
approval from authorities for its use in Japan. But Opdivo became
available not long after its development and has proven to be effective,
according to Amano.
However, he expressed hope that further progress is seen in related studies
due to the high costs required for the treatment, which are said
to top over ¥10 million per person annually.
In his youth, Honjo was not necessarily clear on what path he should take.
After pondering what career to pursue — diplomat, lawyer or doctor —
Honjo entered the Faculty of Medicine at Kyoto University in 1960
and moved on to the graduate course.
During his university days, a fellow student died of stomach cancer,
which led him to think that he would someday like to get involved
in tackling the disease.
After studying for a few years in the United States, where he was exposed
to the latest research on genes and immunology, he decided to return
to Japan and continue his research there, partly because he wanted
to give his children a Japanese education.
In 1992, when Honjo was a professor at his alma mater's faculty of
medicine, one of the members at his laboratory discovered the protein
PD-1, which opened a pathway for a new cancer treatment
that came to be known as immunotherapy.
But at the time, the substance was just a byproduct of an experiment and
no one knew what kind of function it had, according to Yasumasa Ishida,
an associate professor at the Nara Institute of Science and Technology,
who found the protein.
The study was later joined by Nagahiro Minato, an expert on antibodies,
who is now executive vice president of Kyoto University, and
researchers found ways to enhance the human immune system
to attack tumor cells through animal testing and other experiments.
Honjo was “not interested in earning fame,” Minato recalled.
“He probably thought it was his obligation to make medicine that is helpful.”
At the news conference Tuesday, Honjo described his life as “blessed.”
“It has been so fulfilling that I wish I could live this life all over again,”
he said, while thanking his family for allowing him to focus on his research.
Also attending the news conference was his 75-year-old wife, Shigeko,
who said, “I have taken upon myself the job of supporting my husband,
so I am very happy that he received the Nobel Prize.”
Shigeko, who studied math and science at university, said the Nobel Prize
made her think she can now settle down after years of supporting her family
while Honjo went from job to job, forcing their children to change schools.
Fugitive Junya Hida caught in Yamaguchi
Saturday, September 29, 2018
30-year-old fugitive, Junya Hida who escaped from a police station
in Osaka Prefecture in August has been captured in Yamaguchi Prefecture,
western Japan.
Junya Hida was indicted for burglary causing injury and theft and
was served a fresh arrest warrant for attempted sexual assault.
Junya Hida escaped from the Tondabayashi police station on August 12th
by kicking loose an acrylic partition in an interview room after meeting a lawyer.
Some 3,000 police officers were deployed to track him down.
Junya Hida was finally apprehended after he was caught shoplifting
in Yamaguchi Prefecture on Saturday.
Police identified him by his fingerprints and a tattoo on his leg.
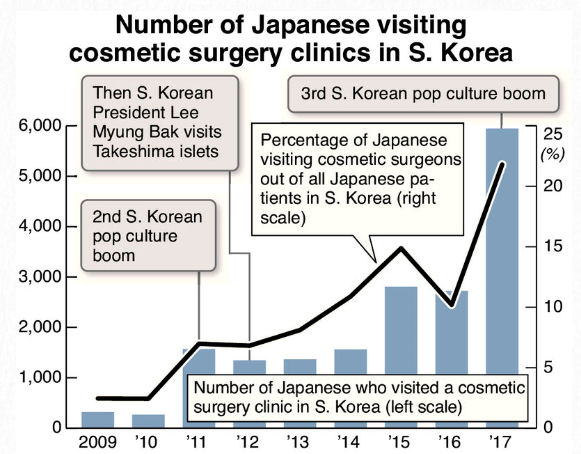 S. Korean cosmetic surgery booming
Saturday, September 29, 2018
S. Korean cosmetic surgery booming
Saturday, September 29, 2018
The number of Japanese people visiting South Korean medical institutions
for cosmetic surgery swelled to about 6,000 in 2017, an almost 20-fold increase
from nine years earlier, according to South Korean authorities.
In addition to South Korea's close proximity to Japan and relatively
cheap surgical costs, the surge in Japanese clients has apparently been inspired
by the popularity of K-pop stars.
However, some clients have also reported post-surgery problems, and
the National Consumer Affairs Center of Japan is urging potential clients
to “think hard” about whether they really need such surgery.
In early September, a first-year university student from Kanagawa Prefecture
traveled to Seoul during her summer vacation. She visited a cosmetic surgery
clinic and had an operation that made the bridge of her nose
three millimeters higher and changed her nose's shape.
“Your facial swelling will go down in about 10 days,” the South Korean
doctor said, according to the 18-year-old student.
“I'd had a complex about my flat nose since I was a junior high school student,”
she said. “I wanted to have confidence in my facial appearance.”
The surgery cost about ¥400,000 ($3,570), around half of what the bill
would have been in Japan. The student decided to have the operation
in Seoul because she admired the face of a member of Twice,
a popular South Korean girl group. She had never been to South Korea
before and does not speak Korean. The student took a liking to a post-surgery
photograph of a nose posted on a blog written in Japanese and decided
to visit the clinic.
Three specialist doctors work at the clinic. Ninety percent of the about 30
clients who visit the clinic each day for surgery or consultations are Japanese.
About 80 percent of them are in their late teens or 20s, and the majority go
under the knife to change the shape of their nose or eyes.
The clinic's reputation has spread through word of mouth, Twitter and other
channels, and its number of customers reportedly has more than doubled
since it opened in 2015.
According to South Korea's Health and Welfare Ministry, 319 of the Japanese
who visited South Korean medical institutions in 2009 did so
for orthopedic surgery such as cosmetic surgery.
In 2011, this figure skyrocketed to 1,570 on the back of the second South
Korean pop culture boom in Japan, in which groups such as Shojo Jidai
(Girls' Generation) and KARA became sensations.
The third wave of the South Korean boom in 2017 pushed this number
up to 5,947 people — about 19 times the 2009 figure. The number of Japanese
clients is hugely influenced by the popularity of South Korean pop culture.
In South Korea, many celebrities and well-known personalities
have openly admitted to undergoing cosmetic surgery.
According to sources, many Japanese clients show doctors images of
popular idols and say, “I want my face to look like this.”
China accuses US of
'trade bullyism'
Tuesday, September 25, 2018
China has strongly accused the administration of US President Trump
of "trade bullyism" against the country.
China's government released a document consisting of some 36,000
Chinese characters on Monday, the day the United States imposed
the largest-ever round of tariffs on imports from China.
The document says that since Trump took office in 2017, the US
administration has brazenly preached unilateralism, protectionism
and economic hegemony.
It stresses that the US has repeatedly intimidated other countries,
particularly China, through economic measures such as tariffs.
It also says that if prices of cheap Chinese imports, which
have been supporting the US economy, rise as a result of
the sanctions, it will hurt the interest of US consumers.
The document suggests the US will be harmed by its own sanctions
as a result of additional costs for manufacturers, resulting in fewer jobs.
It also denies accusations that China is unfairly obtaining US
technologies, saying it is committed to the rule of international law.
It adds that China is protecting intellectual property rights as well as
the interests of foreign firms in the country.
But the document also says cooperation is the only correct option
for China and the United States.
China: Half of firms will be facing US levies
Thursday, September 20, 2018
China's Commerce Ministry says that almost half of the companies
to be affected by new US tariffs on Chinese goods would be foreign firms.
Commerce Ministry spokesperson Gao Feng made the remark at a news
conference on Thursday.
The administration of US President Donald Trump on Monday announced
additional tariffs of 10 percent on about 200-billion dollars' worth of
Chinese imports. The measure will take effect on September 24th.
Gao Feng said companies expected to be affected by the new tariffs
would mainly be from 6 industries in China, such as machinery and textiles.
He said almost half of these firms would likely be foreign companies.
He criticized the US move, saying the administration's protectionism will
cause damage to the global supply chain, as well as the interests of firms
and consumers in the US and China.
Gao Feng said China will support companies facing difficulties with trade.
He indicated the government will help exporters through measures
including reducing the time and cost required for customs procedures.
Mangkhut hits southern China
Monday, September 17, 2018
Severe tropical storm Mangkhut is weakening after making landfall
in southern China, and is now moving west.
In Guangdong Province, the deaths of 2 people have been confirmed.
Nearly 300 people were reportedly injured in Hong Kong alone.
China's weather authorities say the storm approached Hong Kong
after pounding the Philippines' Luzon Island.
They say it made landfall in Guangdong Province on Sunday evening.
China's state-run media are reporting that the storm caused severe damage
to houses and major blackouts.
About 250,000 people reportedly fled their homes in the Guangxi Zhuang
Autonomous Region.
Chinese weather officials say localized downpours could occur in areas
in the storm's path and is calling on people to stay vigilant against heavy
rain and strong winds.
Naomi Osaka Speaks at News Conference
Saturday, September 15, 2018
Fresh off her historic US Open win, tennis star Naomi Osaka is back in Japan.
She arrived Thursday morning and spoke to a large crowd of reporters.
She began by thanking her supporters and expressed sympathy toward people
affected by the Hokkaido earthquake. "I really appreciate all the support.
And also I want to say I heard about the stuff that happened in Hokkaido
so I really wish that everyone is really safe and I send all my wishes."
Osaka's win was overshadowed by Serena Williams' frequent outbursts
during the match over what some see as an unfair umpire.
But Osaka says it didn't take away from accomplishing a lifelong dream.
"I don't feel sad because I wouldn't even know what I'm expected to feel,
because I feel like since it was my first final and first grand slam Victory,
overall I'm happy and I accomplished a lot."
She said she hopes tennis will become more popular in Japan based on the success
of her and a male Japanese star, Kei Nishikori. "Definitely I was thinking
about if little kids were watching, they'd want to play tennis too.
I've always thought Kei is a super good role model on the men's side and
I wish there was one on the women's side, so hopefully I can be that role model."
During the press conference, she also addressed fans in her basic Japanese,
saying, "I like sushi....and thank you for cheering for me!”
Hurricane Florence wreaks havoc in US
Saturday, September 15, 2018
Hurricane Florence is triggering severe flooding and blackouts
after making landfall in the US state of North Carolina.
US weather officials said the powerful hurricane made landfall
in the state on Friday.
The hurricane has been downgraded to a category 1 storm,
the lowest on the 5-point scale, but the officials say it maintains its strength.
Hurricane Florence has a central atmospheric pressure of 968 hectopascals
and is packing winds of up to 126 kilometers an hour.
The officials say more than 1,000 millimeters of rainfall and storm surges
exceeding 3 meters are possible in some areas until early next week.
Washington DC and 5 states including North and South Carolina
have declared a state of emergency. More than 1 million people have been
told to evacuate.
The hurricane knocked down roadside trees in North Carolina.
Police said a tree hit a house, killing a mother and her baby.
Storm surges and heavy rainfall have flooded streets. Some houses
are inundated. Blackouts have affected over 500,000 households
in North and South Carolina.
Powerful hurricanes last year killed many residents in Texas and
the US territory of Puerto Rico.
Putin: Peace treaty first, N. islands later
September 13, 2018, Yomiuri English News
VLADIVOSTOK, Russia — Russian President Vladimir Putin proposed
Wednesday that Russia and Japan sign a peace treaty by the end
of the year without preconditions.
The proposal means that resolving the bilateral issue on the northern
territories would effectively be put aside and a conclusion of the peace treaty
would happen first.
Putin made the proposal during a plenary session of the Eastern
Economic Forum in Vladivostok, Russia, in response to a question
from a coordinator after Prime Minister Shinzo Abe delivered a speech.
Social security cost hit record at over $1 tril.
September 1, 2018, Saturday
Japan's social security costs hit record high in fiscal 2016 of more than
one trillion dollars as the population continues to age.
The National Institute of Population and Social Security Research says
the expenditures from April 2016 through March 2017 stood at almost
117 trillion yen, or about one trillion dollars. That's up 1.3 percent
from a year before.
Pension payments stood at 490 billion dollars, while medial costs were
346 billion dollars.
Welfare spending such as nursing care for the elderly and welfare
benefits was 217 billion dollars.
The social security spending translates into 8,300 dollars per person.
The institute says that in addition to the population aging, advanced
medical care and one-off benefit payments to low-income senior citizens
contributed to the increase.
The country's social security spending has been setting a new record
in recent years.
The government projects that the spending will exceed 1.7 trillion dollars
by fiscal 2040.
The Changing Japanese Household
August 29, 2018, Wednesday
The father goes out to work and the mother stays at home to take care of their two kids
-- this is what Japan’s Statistics Bureau once called a "typical household."
For years, this standard family unit was used as the criterion for compiling
macroeconomic statistics and designing tax systems.
But now, it represents fewer than 5% of all households, due to Japan's
demographic shifts, including a rapidly aging population and declining birthrate.
Defining the "typical household”
According to the Bureau’s definition, a "household" consists of one or more
people who share their residence and live off a common budget.
The bureau started using the term "typical household" in the 1970s in its survey
on household income and expenditures, a closely watched economic indicator.
The Finance Ministry also uses the concept of a “typical household” as a model
for calculating the impact of tax or social security reforms.
"Families consisting of a father who is a salaried worker and a stay-at-home
mother with two children were the norm in Japan during its era of high
economic growth and urbanization (in the 1970s)," says Shungo Koreeda of
the Daiwa Institute of Research (DIR).
"The government and businesses thought the most realistic way to analyze Japan's
situation and plan systems for the future was to base their discussions
on such model households."
Changing standards
But the term “typical household” used for so many years diverges
from the social reality of today.
In 1974, right after the end of Japan's high-growth years, families comprising
one breadwinner and a homemaker were the most common kind of household
types at 14.6% of the total, according to an estimate by the Daiwa Institute
of Research.
But they slipped to 2nd place with 9.7% in 1988 during Japan's bubble
economy, and to 9th place with 4.6% in 2017.
In light of such changes, the Statistics Bureau dropped the term "typical
household" in 2004. Now it defines such units as "a 4-member household
with one earner," a change in wording that underscores an awareness
that such households are no longer "typical."
Households become more diverse
The shift is marked by a steady rise in the number of single-person households.
In 2017, units with one person with no job were the most common group,
accounting for 16.9% of the total. Households consisting of a single earner
stood at 15.6%. Together, they made up a third of all households in Japan.
As for the first group, the aging population means more households are
living off pensions, Koreeda says. And many of the "single earners" are
women who have entered the workforce and marrying later in life.
An increase in the number of divorced couples is another factor.
In the meantime, the number of households with no earners are increasing.
They accounted for a mere 6.8% in 1974, but rose to 32.3%, nearly a third,
by 2017.
The trend is certain to continue -- and at a faster pace. The National Institute
of Population and Social Security Research estimates that the ratio of
single-member households is expected to reach 39% by 2040.
The institute also projects that 40% of households headed by people aged
65 or older will become single member units. It says 42%. will be headed
by people aged 75 or older.
The household concept under review
Koreeda says the current social security and tax systems were devised
using "typical households" as a model, and so many of their measures are
out of synch with the country's demographic profile.
"For instance, the government gives a tax break for a salaried worker
whose spouse is a full-time housewife but officials have debated scrapping
the measure, because there are now more double-income couples,” he says.
As Japan's population ages, the birthrate declines, and the population
becomes more diverse, officials may soon need to pay a lot more attention
to policies that meet the needs of people living alone.
Manga-artist Momoko Sakura has died
August 28, 2018, Tuesday
The Japanese manga-artist famous for her character Chibi Maruko-chan
has died. Her office says she passed away from breast cancer on August 15th.
She was 53 years old.
Momoko Sakura from Shizuoka Prefecture made her debut as a manga artist
in 1984.
In 1986, she started to draw the Chibi Maruko-chan series, modeling
the character after her childhood self.
In 1990, a commercial TV broadcaster began airing an animated version
of the series. Nearly 30 years on, the program is still on the air and is
one of the most popular TV series in Japan.
Sakura wrote the lyrics for the show's closing theme song, which became
a big hit. Her essays also became best sellers.
On Monday, her office rereleased a comment Sakura made in 2014 when
she marking 30 years in the business.
In it, she said she had experienced many good things and many challenges
over the past 30 years. She said she had led a happy life as an author and
was thankful for that.
Japan's Ikee gets 6th gold medal
at Asian Games
August 24, 2018, Saturday
On the 7th day of the Asian Games in Jakarta, Indonesia,
Japan won 12 medals, including 4 gold.
18-year-old swimmer Rikako Ikee won her 6th gold medal,
setting a new Japanese record.
Ikee finished first in the women's 50-meter free style on Friday.
She said she felt more nervous than in any of her previous events
in the Games, but added that she used all her remaining strength
to get her 6th gold.
In the men's 50-meter breaststroke, Yasuhiro Koseki finished first,
clinching his 3rd gold in that event.
Japan also set a new record in softball, winning in 5 straight Asian Games.
Japanese softball players defeated their Taiwanese rivals in the final,
beating them seven-nil in a called game.
US, China slap extra tariffs
on each other's goods
August 23,2018, Thursday
A new round of US tariffs on Chinese imports kicked in on Thursday,
with additional duties of 25 percent on 16 billion dollars' worth of Chinese
products. China responded immediately with tariffs on the same scale.
Trump's administration says the step, based on section 301 of the US Trade Act,
is in response to unfair transfers of US technology and intellectual property to China.
The US is collecting extra duties on 279 items, including plastic products,
semiconductors and optical fibers.
Combined with the punitive tariffs that took effect in July, 50 billion dollars'
worth of Chinese imports are now subject to extra duties.
China has responded with tariffs on the same scale.
The Chinese measures target 333 US goods, including aviation fuel,
diesel vehicles and medical equipment.
In an interview with CNBC, US Commerce Secretary Wilbur Ross said
the US economy is surging and he doesn't feel that the US as a country is being
bothered very much by Chinese retaliations.
The Trump administration is also preparing to slap higher duties on
an additional 200 billion dollars' worth of Chinese imports.
US and Chinese officials began talks in Washington on Wednesday,
resuming the formal trade negotiations that had been stalled since June.
But it's not clear whether the talks will lead to any solutions amid
the escalating trade friction.
Don Quijote 'interested' in buy
troubled Seiyu from Walmart
August 19, 2018, Sunday
The president of discount retailer Don Quijote Holdings Co. expressed interest
Monday
in buying supermarket chain Seiyu GK from Walmart Inc.
"I'm interested if Walmart is serious about selling," Koji Ohara
said
during a business strategy forum in Tokyo.
" It depends on the content, but if it's selling I'd like to carefully
examine the conditions."
Sources close to the matter said last month that Walmart has decided to
sell the troubled
supermarket chain and had already been in talks with some retailers and
investment
funds.
Walmart forged a capital tie-up with struggling Seiyu in 2002, and made
the Tokyo-
headquartered chain a wholly owned unit in 2008. Seiyu has had a tough
time turning
its business around despite restructuring efforts, including job cuts and
closures of
unprofitable outlets, amid intense competition from online retailers.
A sale would be Walmart's latest exit from a lower-growth market as it
looks to shake
up its overseas business and invest in places like China and India.
The world's biggest retailer said last month it had sold an 80 percent
stake in its
Brazilian operations to private equity firm Advent International, exiting
.
an underperforming business in its third major international deal since
April.
In addition to competition from online retailers such as Amazon.com, Japan's
superstores are being squeezed by alternative chains such as convenience
stores
and discount drugstores in a sluggish consumption environment.
Walmart struggled to replicate the success of its low-price model with Seiyu,
despite the introduction of an "everyday low price" pledge and
frequent discounting.
Newspapers across US slam Trump
August 17, 2018, Thursday
Newspapers across the United States have carried editorials protesting
President Donald Trump's assault on media outlets critical of him.
Trump criticizes such media organizations in public speeches and on his
Twitter account, describing them as fake news and the enemy of the people.
More than 300 newspaper publishers expressed a protest in their editorials
in print and online editions on Thursday.
The Boston Globe had called on other publishers to carry such columns.
The paper's editorial is titled "Journalists are not the enemy."
The editorial says "Lies are antithetical to an informed citizenry,
responsible for self-governance." It says "The greatness of America is
dependent on the role of a free press to speak the truth to the powerful."
The article concludes that "To label the press 'the enemy of the people' is
as un-American as it is dangerous to the civic compact we have shared for
more than two centuries."
China to become 2nd largest contributor to UN
August 15, 2018,Wednesday
China is expected to replace Japan as the 2nd largest contributor to the United Nations' general budget.
The world body on Tuesday released a report on calculations of contributions
for the 3-year period
from 2019 to 2021.
Every 3 years, the UN reviews each member nation's budget share based on
economic indicators
including gross national income and other factors.
The latest UN report shows the United States will remain the largest contributor
at 22 percent.
China is expected to become the 2nd largest contributor at 12.01 percent, followed by Japan
at 8.56 percent.
For the period from 2016 to 2018, Japan was the 2nd largest contributor
with a share of 9.68 percent,
followed by China's 7.92 percent.
The new budget share is expected to be determined by the General Assembly by the end of this year.
Many observers say China's influence in the UN will likely grow as the
administration of
US President Donald Trump is reconsidering its cooperation with the world
body.
Observers also say Japan will need to promote its presence by stressing its contributions
to international and diplomatic activities at the UN.
Japan aims to improve relations with China
August 13, 2018, Monday
Yesterday marks the 40th anniversary of a landmark friendship treaty
between Japan and China. The Japanese government plans to improve
bilateral ties with China, and hopes that Prime Minister Shinzo Abe
will visit China by the end of the year.
The Japan-China Treaty of Peace and Friendship was signed on August 12th,
1978. It calls for respecting each other's sovereignty and territory and
promoting friendship.
Bilateral relations have improved recently, with dialogue between leaders
and at ministerial levels.
Officials from the 2 countries met on the sidelines of a foreign ministerial
meeting in Singapore earlier this month.
They agreed that relations are back on a normal path and to move forward
with mutual visits by their leaders.
The Japanese government hopes that Prime Minister Shinzo Abe will visit
China this year, and that Chinese President Xi Jinping will visit Japan
at a later date.
Japan also plans to accelerate the building of a new cooperative economic
relationship. This would include a meeting of a new public-private
forum aimed at promoting cooperation between Japanese and Chinese
businesses in third countries.
The Japanese government says it also wants to continue dialogue
to resolve such issues as China's gas field development in the East China
Sea and Chinese vessels entering Japan's waters around the Senkaku Islands.
Japan controls the islands in the East China Sea. China and Taiwan
claim them. The Japanese government maintains the islands are
an inherent part of Japan's territory, in terms of history and international law.
Bilateral relations soured when the Japanese government purchased some
of the Senkaku Islands in Okinawa Prefecture from their Japanese owner in 2012.
50 Chinese ships entered Japanese waters
so far in 2018
August 12, 2018, Sunday
Japan Coast Guard officials say that, so far this year, 50 Chinese patrol ships
have been spotted in Japanese territorial waters near the Senkaku Islands
in the East China Sea. They say the sightings occurred over a total of 14 days.
Japan controls the islands. The Japanese government maintains they are
an inherent part of Japan's territory. China and Taiwan claim them.
Officials say such sightings have occurred since 2012, when Japan's
government acquired ownership of some of the islands from a Japanese owner.
In 2017, a total of 108 Chinese patrol vessels entered the waters over a total
of 29 days.
Coast Guard officials say if further incidents take place, they will continue
to demand the vessels immediately leave Japanese waters and take
firm action in accordance with international and domestic laws.
N.Korea criticizes UN chief's remarks
World News 2018.8.11,
Saturday
North Korea has criticized UN Secretary-General Antonio Guterres for making
"reckless remarks" about the country.
The North's mission to the United Nations issued a statement on Friday.
Guterres has said that North Korea can be a "normal member" of
the international
community through total denuclearization that is verifiable and irreversible.
He made the statement during a joint news conference with Japanese Prime Minister
Shinzo Abe in Tokyo on Wednesday.
He also pledged his full involvement as UN chief in UN Security Council
resolutions that
include sanctions against the North.
The mission criticized Guterres' remarks for coming at a time when the
world supports
and welcomes the historic North Korea-US summit and joint statement in
Singapore.
The mission is calling on Guterres to do something beneficial for peace and stability
on the Korean Peninsula instead of siding with sanctions that only please some countries.
The North's mission has repeatedly condemned UN Security Council resolutions
in the past.
But it is rare for the mission to criticize the UN chief by name.
Medvedev warns of 'economic war'
World News, 2018.8.11, Saturday
Russian Prime Minister Dmitry Medvedev has suggested that
Russia will take countermeasures if the United States expands sanctions.
Medvedev said on Friday that if a penalty such as a ban on banking
operations or the use of currency follows, it would amount to a declaration
of economic war.
He said it would be necessary for Russia to respond with economic, political,
or other means.
The US administration said on Wednesday that it would impose new sanctions
on Russia over its alleged involvement in a nerve-agent attack against
a former Russian spy in Britain in March.
The US says it will ban the export to Russia of turbine engines, electronic
components and other products that could be used for weapons.
After the announcement, Russia's currency, the ruble, tumbled.
It hit a 2-year low against the dollar at one time on Friday.
Heat wave sends mercury
to all-time high of 41.1 in Saitama
July 24, 2018, Tuesday
Japan recorded its highest temperature ever Monday as the mercury
hit 41.1 in Kumagaya, Saitama Prefecture, amid a deadly heat wave,
the Meteorological Agency said.
Scorching weather killed 77 people and sent more than 30,000 people
to hospitals across the nation from July 9 to Sunday, according to
the Fire and Disaster Management Agency and a Kyodo News tally.
The heat wave also set a new record in Tokyo, where a sizzling
temperature of 40.8 degrees was logged in Oume, the agency said.
The temperature in Kumagaya hit 41.1 degrees at 2:16 p.m.,
eclipsing the previous record of 41.0 set in August 2013 in Shimanto,
Kochi Prefecture.
The temperature also hovered near a record high in Tajimi, Gifu
Prefecture, which saw the mercury touch 40.5 on Monday after hitting
40.7 on Wednesday, the agency said.
The heat wave has been baking the nation for weeks while survivors
of the rain disaster in western Japan continue to recover from fatal
floods and hundreds of land slides earlier this month.
Despite warnings about the heat across the nation, at least five people
— all senior citizens — died in prefectures around Tokyo
from Sunday to Monday, local authorities said.
The victims included a 90-year-old man in Chichibu, Saitama Prefecture,
an 89-year-old man in Namegata, Ibaraki Prefecture, and a 95-year-old
woman in Sano, Tochigi Prefecture whose deaths are thought to have been
caused by heatstroke.
The other two, who also died in Saitama, were an 85-year-old woman
in Kuki and a 72-year-old woman in Asaka.
The Tokyo Fire Department dispatched ambulances 3,125 times on Sunday
alone, the largest figure for a single day since it began emergency services
in 1936, apparently due to a surge in the number of people falling ill
from the intense heat.
As of Monday, the number of people who have been taken to hospitals
by Tokyo's ambulances due to heatstroke this year stood at 3,544,
already surpassing the 3,454 for the whole of last year,
according to the department's preliminary report.
The intense heat has prompted the Meteorological Agency to issue
advisories urging people to drink water frequently and take measures
to prevent heatstroke.
At a news conference Monday, the agency said the heat wave is expected
to continue into early August in both western and eastern Japan.
“Please be aware of heatstroke, and make sure to keep yourself hydrated
and consume adequate salt,” an agency official said.
Japan's summers are notoriously hot and humid, and hundreds of people
die each year from heat-related illnesses, particularly the elderly.
But this year's scorching weather came as a surprise and has since revived
safety concerns about the 2020 Olympics, which will be held in July and
August, typically the hottest months of the year.
Olympic officials and the Tokyo Metropolitan Government are examining
such measures as solar-blocking road paint and mobile misting stations
to reduce the heat.
Severe heat continues across much of Japan
July 21,2018, Saturday
Temperatures on Saturday are expected to reach 38 degrees Celsius
in some parts of eastern and western regions.
People are advised to remain on the alert to prevent heatstroke.
In Kyoto, Friday's daytime high reached 38.6 degrees.
It was the first time since record-keeping began 138 years ago
that temperatures in the city hit the 38-degree mark on 7
consecutive days.
Many people have been taken to hospital, while others were found
dead in recent days, apparently stricken by heatstroke-related
conditions.
Health experts are urging people to use air conditioners,
drink water, and take in salt, as well as to refrain from
daytime exercising.

Starbucks shuts 8,000 shops for anti-bias training
May 30, Wednesday,2018
Starbucks has temporarily closed about 8,000 of its directory managed
coffee shops across the United States to give employees anti-bias training.
The move follows an incident last month where 2 black men were arrested
while waiting for a friend at a Starbucks cafe in Philadelphia, Pennsylvania.
An employee had called police, saying the men refused to make a purchase
or leave.
The arrests sparked accusations of racial profiling and nationwide protests
after a video of the incident was uploaded on Twitter. The coffee chain
issued an apology.
A total of 175,000 employees took part in a 4-hour training session
on Tuesday. They heard experts on racial tolerance and talked about
unconscious bias and unintentional discrimination.
Starbucks also published a full-page advertisement in the New York Times
and other media on Tuesday, in which Chairman Howard Schultz pledged
to prevent a recurrence of the problem.

Japanese movie wins top prize at Cannes
May 21, Monday, 2018
Japanese Director Hirokazu Koreeda's "Shoplifters" has won
the Palme d'Or at the Cannes Film Festival.
At the 71st edition of Cannes, Koreeda's work was one of 2 films
by Japanese directors in competition for the Palme d'Or.
The other one was "Asako I & II" directed by Ryusuke Hamaguchi.
On the last day on Saturday the head of the jury, Actress Cate Blanchett,
announced Koreeda's victory.
It was Koreeda's 5th time to be in competition for the Palme d'Or and
his first in 3 years. In 2013, his "Like Father, Like Son" won the jury prize.
"Shoplifters" is about family ties. It's a story of a Tokyo family
that lives on the pension benefits of one of its members.
They shoplift when they are really hard up.
The last Japanese film to win the top honor at the festival was 21 years ago
-- director Shohei Imamura snatched the Palme d'Or in 1997 for his film "The Eel."
Cannes is one of the 3 most prestigious film festivals in the world,
the other two being Berlin and Venice.

Japan's current account surplus grows again
May 10, 2018
Japan's finance ministry has announced that the country's current account balance
for fiscal 2017 was in surplus, growing for the fourth consecutive year.
The current account is the broadest measure of trade and investment with the rest
of the world.
The finance ministry says the surplus stood at about 198.5 billion dollars,
a gain of more than 6.6 billion from the previous year.
The number is the third largest on record.
The primary-income account gauging earnings from overseas investment
also expanded to show a surplus at about 181.8 billion dollars.
The surplus in the travel account was about 17 billion dollars, a record high.
The trade surplus came in at about 41.8 billion dollars.
The figure was lower than the previous year, mainly due to a rise in the value
of imports, such as crude oil and liquid natural gas.
Four Big Pollution Diseases of Japan
The four big pollution diseases of Japan were a group of man-made
diseases all caused by environmental pollution due to improper handling
of industrial wastes by Japanese corporations. The first occurred in 1912,
and the other three occurred in the 1950s and 1960s.
Due to lawsuits, publicity, and other actions against the corporations
responsible for the pollution, as well as the creation of the Environmental
Agency in 1971, increased public awareness, and changes in industrial
practices, the incidence of these kinds of diseases declined after the 1970s.
April 12, Thursday, 2018

Pollution in China and S.Korea
South Korea President Moon Jae-in said on Friday cooperation
with China is needed not only to end tension over North Korea's
weapons but also to tackle the problem of air pollution.
Moon raised South Korea's concern about pollution arriving from China
in talks with Chinese special enjoy Yang Jiechi, in Seoul to brief
South Korea on this week's meeting between Chinese President Xi Jinping
and North Korean leader Kim Jong Un.
"President Moon Jae-in stressed the seriousness of South Korea's fine dust
problem," said South Korea's presidential spokesman, Kim Eui-kyeom.
"Korea's fine dust is caused by factors at home but as a Chinese factor
also lies behind the problem, voices are growing among South Koreans
asking for close cooperation between South Korea and China,"
the spokesman cited Moon as saying.
Worsening air pollution has been a chronic problem in South Korea
especially during the spring.
Authorities have suspended operations at five old, coal-fired plants
from March to June to cut pollution.
Yang suggested the two sides work to cut pollution by setting up
a joint environmental center, the spokesman said.
April 11, Wedesday, 2018
Domestic transaction of Virtual currency was
69 trillion yen in 2017
Virtual currency, also known as virtual money, is a type of unregulated,
digital money, which is issued and usually controlled by its developers
(Bitcoin is an exception), and used and accepted among the members
of a specific virtual community.
Japan's Domestic transaction of Virtual currency was 69 trillion yen in 2017
The Financial Crimes Enforcement Network (FinCEN), a bureau of
the US Treasury, defined virtual currency in its guidance published
in 2013.
In 2014, the European Banking Authority defined virtual currency
as "a digital representation of value that is neither issued by a central bank
or a public authority, nor necessarily attached to a fiat currency,
can be transferred, stored or traded electronically".
By contrast, a digital currency that is issued by a central bank is defined
as "central bank digital currency".
Re:
マネジメント&マーケティング
The Transformations driven by the Fourth Industrial Revolution
from World Economic Forum
 Samsung chief Lee Jae-yong
Samsung chief Lee Jae-yong  Merck & Co.
Merck & Co. 






















 (日本某超有名収監試・秘報・日本の二人は、秘酔しても、オンリ-5億円!)
(日本某超有名収監試・秘報・日本の二人は、秘酔しても、オンリ-5億円!)


















































 For historic 2nd time
For historic 2nd time 

























































































