to Former page to Next page
2.1.c Specimens for analyses
The specimen for analyses was caught by a net from surface of the liquid that is shown in Fig.8(c), because the floating materials on the surface escape from the spoon of stainless steel. The substance on the glass wall is the membrane that brings a structural color. Fig. 9 (a) and also Fig. 9 (b) are photographs of dried specimen on a laboratory dish.
The photograph captured by an optical microscope is shown in Fig.10 (a). We can recognize tow types of compounds. One is black particles. And the other is yellow membrane of transparency. The photograph captured by a scanning electron microscope (SEM) is shown in Fig.10 (b).
(a) dried floating materials (b) dried floating materials(expanded)
Fig,9 Dried floating materials made from mixing carbonated water and steel wool
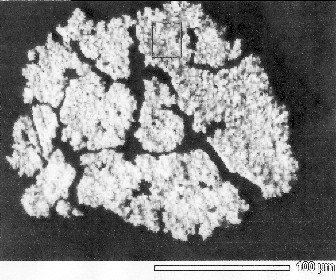
(a) pictured by an optical microscope(x600) (b) pictured by a SEM
Fig.10 Photographs on specimen those were captured by microscope
2.2. Infrared spectroscopic analysis
2.2.a The principle of infrared spectroscopic analysis
What type of chemical bond is found in the material on surface of the water? The molecular bonds vibrate depending on the type of bonds. So, the infrared spectroscopy (Fourier Transform Infrared Spectroscopy: FT-IR) is used as a tool to identify chemical bond. At first, the identification is difficult in case of unknown specimen. But, the absorption peak of vibration due to expansion and contraction on the connection of carbon atom and hydrogen atom in paraffin is in 3000 cm-1. We can check whether or not the absorption peak turns up.
-2.2- to Index