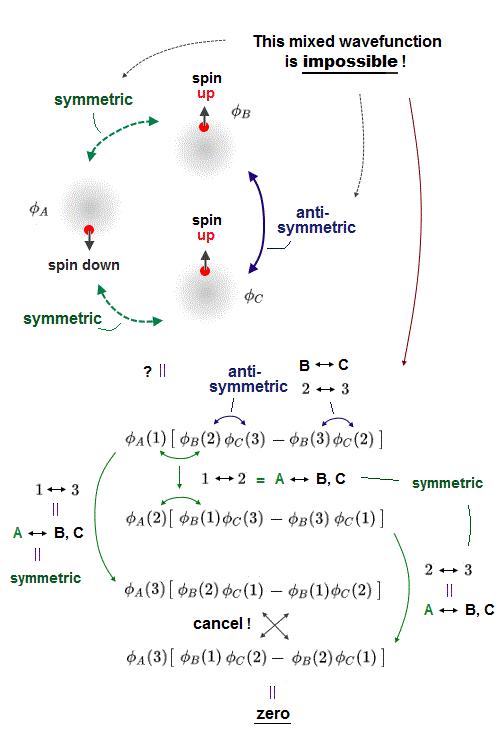
(Fig.1) Mixed antisymmetric (= between two same-spin electrons ) and symmetric (= between two opposite-spin electrons ) wavefunctions is impossible. → Quantum mechanical Pauli principle is wrong.

In order to describe actual interactions between molecules, quantum mechanical wavefunctions have to incorporate Pauli repulsion expressed by antisymmetric wavefunction (= between two electrons with the same spin ) and molecular bond attraction expressed by symmetric wavefunctions.
↑ But this mixed antisymmetric and symmetric wavefunctions, which should explain Pauli repulsion and molecular bond attraction at the same time, are intrinsically impossible to make, so quantum mechanical wavefunctions cannot describe actual Pauli repulsion and molecular bond attraction at all. → Quantum mechanics is wrong.
In the upper figure, φA wavefunction has the down-spin, and the other two φB and φC wavefunctions are supposed to have the up and up spins.
Hence, Pauli repulsion must occur between two electrons with the same up-up spin = two wavefunctions φB (= up-spin ) and φC (= up-spin ) must form Pauli repulsive antisymmetric wavefunctions flipping the sign by exchanging two electrons' variables belonging to φB and φC (= φB ↔ φC ).
Molecular attractive exchange energies must occur between two electrons with the opposite up-down spins = φA (= down-spin ) and the other two φB (= up-spin ), φC (= up-spin ) must form symmetric wavefunctions whose sign don't change under the exchange of two electrons' variables (= φA ↔ φB or φA ↔ φC ).
In the upper figure, the 1st equation is antisymmetric wavefunction which flips the sign by exchanging two electrons 2 and 3 (= corresponds to exchanging φB with up-spin and φC with up-spin ).
Exchanging any two electrons existing in two symmetric wavefunctions φA with down-spin and φB (= or φC ) with up-spin must keep the same sign of their wavefunctions.
For example, exchanging two electrons 1 ↔ 3 in the 1st wavefunction means exchanging two electrons of symmetric wavefunctions between φA with down-spin and φB (= or φC ) with up-spin, hence, this symmetric wavefunction should not change its sign by this exchange of two electrons.
But after all, this mixed antisymmetric (= between the same-spin electrons ) and symmetric (= between the opposite-spin electrons ) wavefunction is impossible to make due to cancellation of terms necessary to form symmetric wavefunctions, which become zero, as shown above.
In conclusion, quantum mechanics is wrong, because its Pauli antisymmetric wavefunctions cannot describe Pauli repulsion (= antisymmetric ) and molecular bond attraction (= symmetric wavefunction ) at the same time.
In the upper section, we prove the current quantum mechanical Pauli antisymmetric wavefunctions cannot express molecules or atoms with more than two electrons, which mix Pauli exchange repulsion between the same spins (= up-up or down-down ) and (molecular) exchange attraction between the opposite spins up-down.
Lithium has three electrons which should contain two electrons with the same spins (= up-up ) and one electron with the opposite down-spin, according to the quantum mechanics.
So according to the quantum mechanical rule, the Lithium wavefunctions must be a mixed wavefunction, which spacial wavefunction is symmetric under the exchange of electrons-1 with up-spin and electron-2 with down-spin (= exchanging spin wavefunction is antisymemtric ), and the spacial wavefunction is antisymmetric under the exchange of electron-1 with up-spin and electron-3 with up-spin (= exchanging spin wavefunction is symmetric ).
↑ But this quantum mechanical Pauli antisymmetric wavefunction is impossible in the three-electron Lithium atom which should be the mixed symmetric and antisymmetric wavefunction.
For example, this paper about the ground-state Lithium wavefunction ( this p.3-(6) ) includes the contradictory exchange wavefunction where exchanging the 1st and 3rd (electron's) numbers changes the sign ( φ(1,2,3) → -φ(3,2,1) ) or does Not change the sign ( -φ(2,3,1) → -φ(1,3,2) ), which is inconsistent.
Also in another lithium-wavefunction paper ( this p.2-right-(3) ), they exchange only the spin wavefunction of the electron-1 and electron-2 without exchanging the electron-3, which is just the two (= Not three ) electron atom without Pauli repulsion.
As a result, quantum mechanical Pauli principle cannot explain any molecules or atoms with more than two electrons containing up and down spins
So quantum mechanics had to unrealistically discard only the molecular attractive bond exchange energies expressed as the symmetric wavefunctions between the opposite spins, and the resultant Pauli antisymmetric wavefunctions expressed as Slater determinants give only the unneeded Pauli repulsive exchange energies between the same spin (= without molecular bond ! ), which disagreed with the actual molecules.
In this way, all the multi-electron Schrodinger equation with Pauli antisymmetric wavefunctions failed, and the one-pseudo-electron approximation called density functinal theory (= DFT ) remains as the only quantum mechanical calculation tool, which also failed, though.

2022/5/25 updated. Feel free to link to this site.