to Former page to Next page


Fig.24 Effects of calcium carbonate (CaO: 2g) on the carbonated water (50cc) that was mixed with iron powder (5g) and [after 3 days from the start]
4.2 Effects of sodium ion for the production of the bubble
in carbonated water with ion
4.2.a Effects of NaCl to the iron carbide bubble
It is considered that the first life in early earth was born in the sea. The sea water includes salt (NaCl). So, the effect of salt to iron carbide bubble was investigated. Fig.25 (a) is the photograph on the pure iron carbide bubble as a reference. Fig.25 (b) is the photograph of iron carbide bubble in the water in which NaCl (2g) was added.
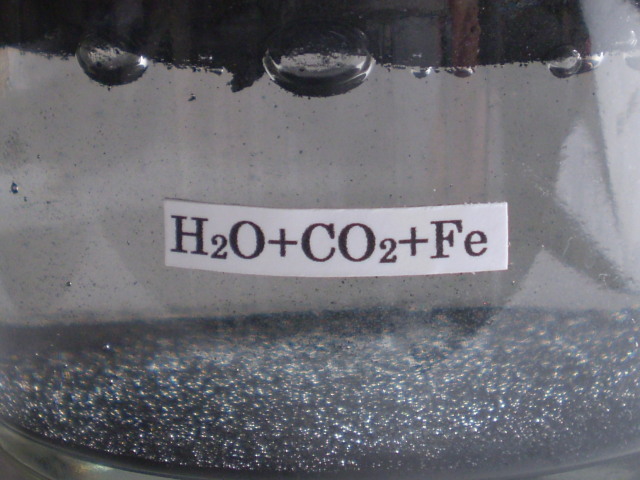
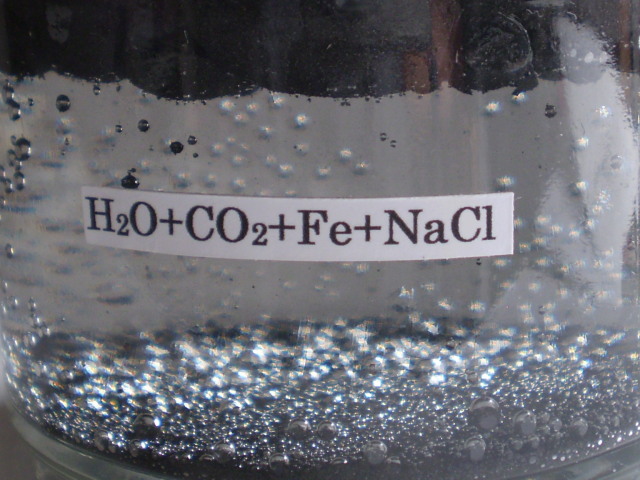
(a) pure iron carbide bubble (b)addition of NaCl (2g)
Fig,25 Effects of salt (NaCl) on the iron carbide bubble
4.2.b Effects of NaHcO3 to the iron carbide bubble
Fig.26 (a) is the photograph of iron carbide bubble in the water in which NaHCO3 (2g) was added. Fig.26 (b) is the photograph of iron carbide bubble in the water in which
NaHCO3 (7g) was added plentifully. The production of bubble changes drastically in the case of (b). It is explained by the acidity of the water. The bicarbonate of soda is weak alkalinity and hydroxyl group of (OH-) exists a lot. The hydroxyl group makes chemical bond with the hydrogen atom and carbon atom. So, the components of the bubble and the membrane disappear.
-4.4- to Index