to Former page to Next page
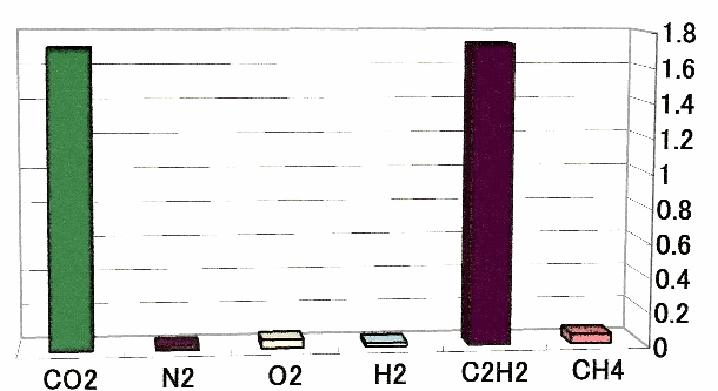
Fig.14 Solubility of typical molecule to the water (1cc) at 0℃ 1atm
The temperature dependency on the solubility of carbon dioxide to the water is shown in Fig. 15 [6]. The solubility of carbon dioxide decreases when the temperature goes up.
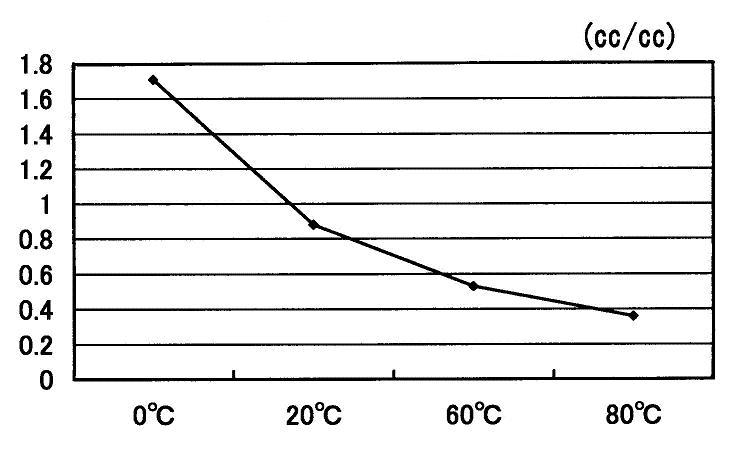
Fig.15 Temperature dependency on the solubility of carbon dioxide to the water
3.1.c Comparison of electronegativity among typical atoms
Electronic state changes at the circumstances. Then, the electronegativity changes by the situation. Even so, the value of electronegativity was given by the results empirically. Comparison of the numerical value of electronegativity indicates how strongly each atom attracts an electron in compound. Fig.16 shows the value of electronegativity given by L. Pauling..
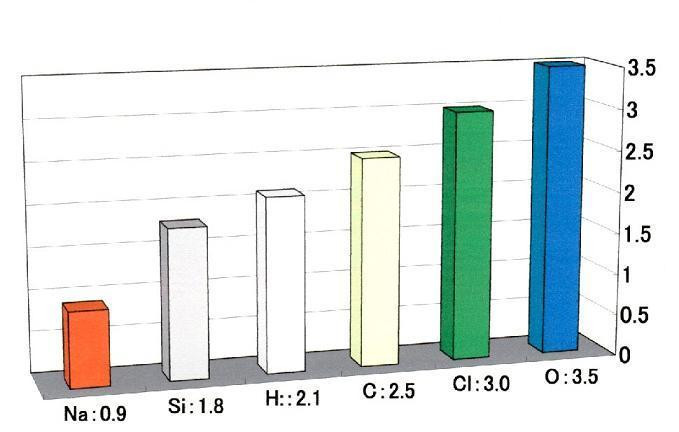
Fig.16 Electronegativity (from L. Pauling)
-3.2- to Index