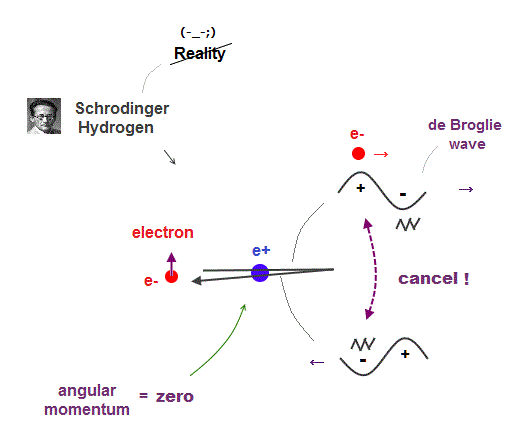
(Fig.1) ↓ Orbital angular momentum of hydrogen is zero !? → crash

Electron spin is unrealistic. Its spinning speed must be faster than light, if an electron is actually rotating.
Then why did this unrealistic spin need to be introduced ?
Schrödinger equation claims hydrogen's electron has zero angular momentum.
It means an electron always crashes into nucleus in quantum mechanics ? Schrödinger equation treats an electron as de Broglie wave.
If an electron's orbital angular momentum is zero, its one-dimensional de Broglie wave interferes with itself destructively !
As a result, quantum mechanics has no choice but to introduce new fictional spin instead of real de Broglie wave.
(Fig.2) Pauli (~ 30 eV ) vs. spin-spin energy (~ 0.0001 eV )
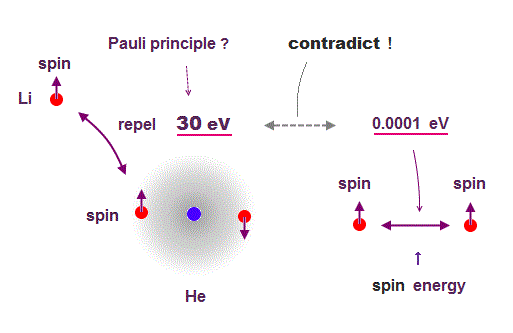
The 3rd electron of lithium is kicked out from inner 1s orbital to outer 2s orbital by Pauli exclusion principle.
Quantum mechanics claims that the electron with the same spin cannot share the same orbital by Pauli force.
But the spin-spin magnetic dipole energy is too weak to explain strong Pauli exclusion energy, so electron spin has nothing to do with lithium 3rd electron.
Spin dipole-dipole magnetic energy is too weak (< 0.0001 eV ).
So, they introduced new artificial nonphysical concept (= spin "exchange" energy ) to explain strong Pauli exclusion ( this p.7 ) !
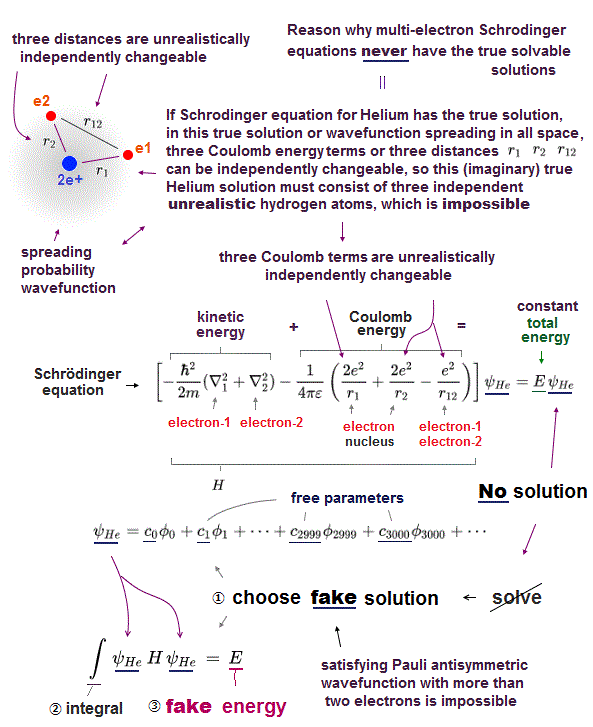
(Fig.3) ↓ If two electrons have the same wavefunction → zero = Pauli ?

Schrödinger equation has NO exact solution in multi-electron atoms, they have to choose wave function as Helium's approximate (= fake ) solution.
They introduced new artificial concept = anti-symmetric wave function as Pauli exclusion force.
The simplist approximate Helium wave function is 2 × 2 determinant form.
If helium two electrons have the same wavefunction with the same spin ( φ1 = φ2 ), the whole helium wave function is cancelled out to be zero ( ψHe → 0 ).
The present physics claims that this nonphysical abstract concept can express actual Pauli principle, which is not physics !
(Fig.4) ↓ Determinant trial wave function means Pauli principle ?
We have to choose fake trial function as an approximate solution of Schrödinger equation in muti-electron atoms with no solution.
After choosing fake trial function from infinite choices, they insert it into Schrödinger equation and integrate it instead of solving.
Repeat the same procedure ( choose trial function → insert → integrate ) until they get their convenient energy E.
So this Schrödinger method has NO ability to predict atomic behavior, useless. Whatever trial functions you choose, they can never be true solution !
They insist that when this trial function is a determinant form (= antisymemtric ), it can express Pauli principle.
But as long as Schrödinger equation cannot be solved, this far-fetched interpretation of Pauli exclusion is meaningless.
(Fig.5) ↓ Lucky coincidence ? Same magnetic moment.
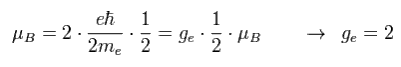
It's known that hydrogen atom has magnetism equal to Bohr magneton, which can be explained by Bohr's classical orbit and de Broglie theory.
After quantum mechanics was born, its Schrodinger wavefunction has No orbital angular momentum to explain this magnetism.
So the physicists at the time invented strange spin, and they artificially defined the spin's magnetic moment as the same Bohr magneton !
This is a very far-fetched interpretation.
Spin's angular momentum is 1/2ħ, which is half of Bohr's ħ angular momentum.
So they decided that spin g-factor is twice (= 2 ) the Bohr's orbit (= 1 ).
"g-factor" means the ratio of magnetic moment to angular momentum.
As a result, they claim spin can also has the same Bohr magneton.
We can only measure the magnetism, neither angular momentum nor g-factor.
The problem is there is No physical reason why "spin" cannot stop and it has the same Bohr magneton.
(Fig.6) Spin-spin magnetic energy is very weak ( < 0.0001 eV ) !
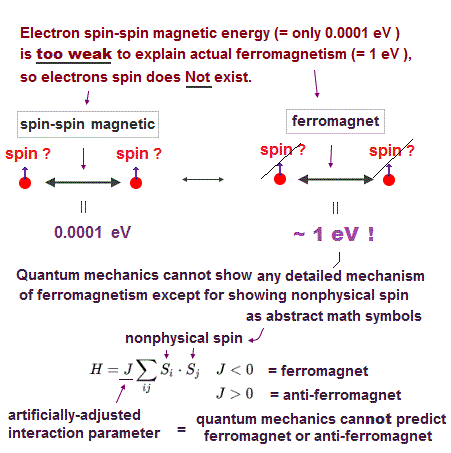
Textbooks often say atomic energy levels can be explained by electron spin singlet-triplet expression. But spin energy is too weak to explain actual experimental results !
Experiments showed that each electron ( spin ) has definite magnetic field. We can calculate spin-spin energy using the equation of magnetic-magnetic interaction.
Spin-spin magnetic energy at the atomic radius ( ~ 1 Å ) is extremely weak (= less than 0.0001 eV, this p.6 ) !
(Fig.7) Singlet state is 1.612 eV higher than its triplet in Mg 3s3p energy level.
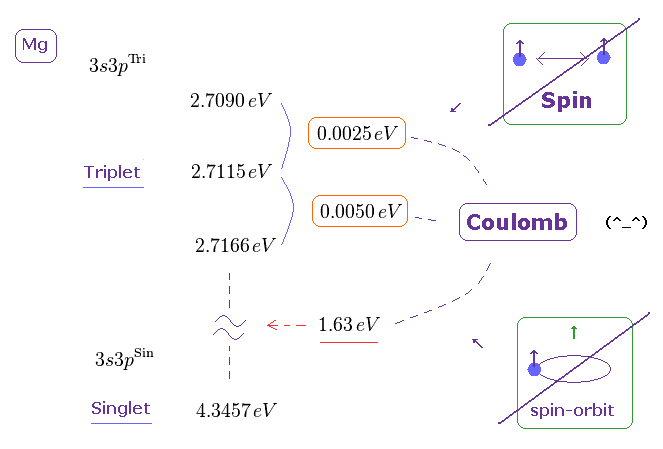
But the actual energy difference between singlet and triplet state is too large to apply weak spin-spin energy in it.
The singlet state is 1.612 eV higher than its triplet state in Magnesium 3s3p ( using energy converter ). But spin-spin magnetic energy is less than 0.0001 eV !
So the singlet-triplet energy levels have nothing to do with unrealistic spin.
(Fig.8) "Spin" is used as "artificial marking" for different energy levels !
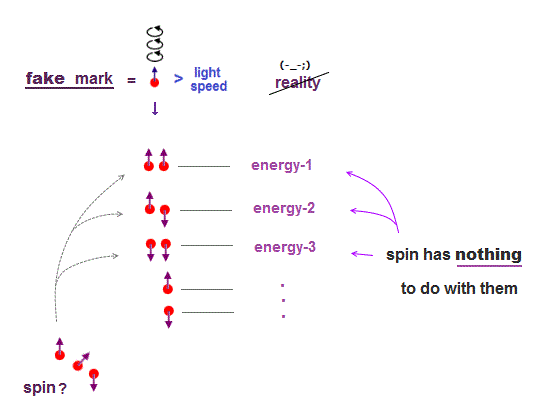
The current basic science has stopped since old quantum mechanics in 1920s. At this time, physicists couldn't describe each energy level by complicated multi-particle Coulomb effect.
This is the reason why they adopted artificial spin as "fictitious mark" to distinguish different energy levels. So spin is not real but just a temporary "tool".
If they couldn't name each different energy level using "fictitious tool of spin", they could have neither published their papers nor proceeded with their academic researches.

2017/6/9 updated. Feel free to link to this site.